- NEWS FEATURE
A reboot for chronic fatigue syndrome research

Elizabeth Allen keeps careful records of the many treatments she has undergone to relieve the symptoms of chronic fatigue syndrome. Credit: Preston Gannaway for Nature
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 553, 14-17 (2018)
doi: https://doi.org/10.1038/d41586-017-08965-0
References
Lombardi, V. C. et al. Science 326, 585–589 (2009).
White, P. D. et al. Lancet 377, 823–836 (2011).
White, P. D., Goldsmith, K., Johnson, A. L., Chalder, T. & Sharpe, M. Psychol. Med. 43, 2227–2235 (2013).
Jason, L. A. et al. Arch. Intern. Med. 159, 2129–2137 (1999).
Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness (National Academies Press, 2015); available at http://go.nature.com/2kydjdi
Nacul, L. C. et al. BMC Med. 9, 91 (2011).
Njoku, M. G. C., Jason, L. A. & Torres-Harding, S. R. J. Health Psychol. 12, 461–474 (2007).
Kapur, N. & Webb, R. Lancet 387, 1596–1597 (2016).
Montoya, J. G. et al. Proc. Natl Acad. Sci. USA 114, E7150–E7158 (2017).
Fluge, Ø. et al. PLoS ONE 6, e26358 (2011).
Tuller, D. ‘Trial by error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study’ Virology Blog (2015); available at http://go.nature.com/2j5fip7
National Institute for Health and Care Excellence. Surveillance report 2017 — Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management (2007) NICE guideline CG53 (NICE, 2017); available at http://go.nature.com/2d4ckro

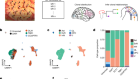
 Biological underpinnings of chronic fatigue syndrome begin to emerge
Biological underpinnings of chronic fatigue syndrome begin to emerge
 Chronic fatigue syndrome: life after XMRV
Chronic fatigue syndrome: life after XMRV