Credit: Rossana Wan
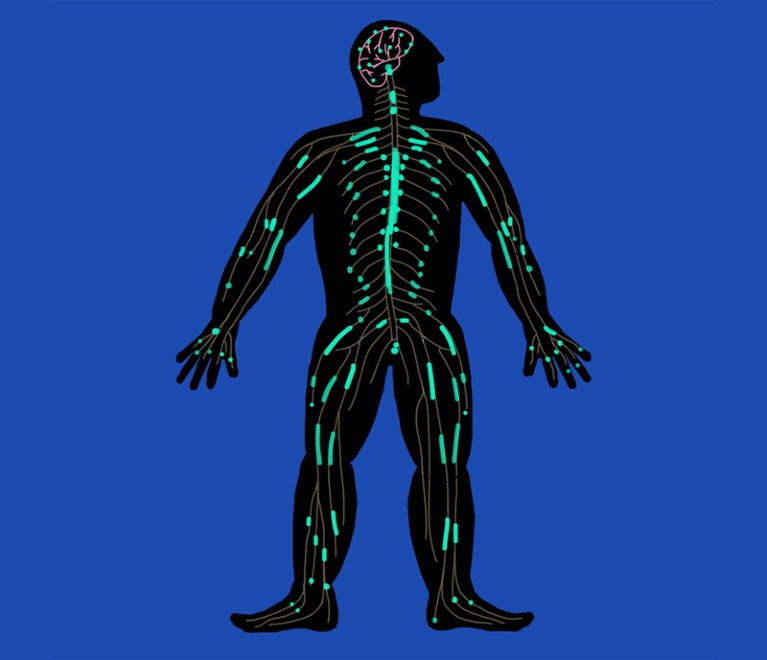
As many as half a million people damage their spinal cord each year. For almost all of them, the injury will be life-changing. The spinal cord is an integral part of the central nervous system (CNS): nerve fibres of the peripheral nervous system project from it, relaying signals to and from muscles and organs — enabling us to move, and to feel heat and pain. But unlike their peripheral cousins, nerves in the CNS very rarely recover from damage.
As a result, injury to any part of the spinal cord can cause a permanent loss or reduction in bodily function below the site of damage. More than half of people with a spinal-cord injury have a ‘complete injury’: loss of function is total, with no motor or sensory function in areas of the body controlled by nerves that emerge from the spinal cord below the damaged site. The remainder of patients have an ‘incomplete injury’: they do retain or recover some function. In both cases, there are no approved ways of repairing the damage, and attempts to bypass the injury through implanted electronics are in their infancy.
There are signs, however, that researchers are on the verge of making crucial breakthroughs. As understanding grows about the complex interplay between different cell types and the extracellular environment at the site of damage, researchers are increasingly optimistic that they will find a way to kick-start the regeneration that is suppressed in the CNS.
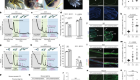
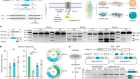
There are three broad approaches. The first focuses on altering the environment around damaged nerves in the spinal cord to remove molecules that are thought to restrict the ability of the nerves to regrow their axons — the long projections that thread through the cord and through which neurons connect to one another. The second aims to intervene in regulatory pathways within the damaged neurons themselves to take the brakes off the cells’ inherent capacity to regenerate.
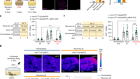
The third approach — and currently the closest to the clinic — deploys mesenchymal stem cells (MSCs). Preclinical studies show that MSCs injected into rats can home in on injured areas of a spinal cord. At the site of injury, the cells seem able to protect neurons from secondary damage caused by immune cells. They can also repair damage to the insulating layer of myelin around axons, promote axon regeneration and assist the formation of new blood vessels — and in so doing, restore some degree of function. They seem also able to differentiate into new nerve cells. The next challenge is to get this to work in humans. The results of a small trial of MSCs in people with a spinal-cord injury are due to be reported soon, and should give an indication as to whether this therapy can progress to larger trials.
The complex factors that prevent the spinal cord from repairing itself might mean that no one approach will be sufficient. It seems likely that a combination of treatments will be needed to support neuronal regeneration and restore function and independence to people with spinal-cord injury.
Nature is pleased to acknowledge the financial support of the Translational Research Informatics Center (TRI) and Sapporo Medical University. As always, Nature retains sole responsibility for all editorial content.

 Nature Outline: Spinal-cord injury
Nature Outline: Spinal-cord injury
 Repairing the neural highway
Repairing the neural highway