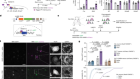
- NEWS AND VIEWS
Early embryos kept in check
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 552, 178-179 (2017)
doi: https://doi.org/10.1038/d41586-017-07436-w
References
Shahbazi, M. N. et al. Nature 552, 239–243 (2017).
Maître, J.-L. Biol. Cell 109, 323–338 (2017).
Frankenberg, S. R., de Barros, F. R. O., Rossant, J. & Renfree, M. B. WIREs Dev. Biol. 5, 210–232 (2016).
Ying, Q.-L. et al. Nature 453, 519–523 (2008).
Shahbazi, M. N. et al. Nature Cell Biol. 18, 700–708 (2016).
Deglincerti, A. et al. Nature 533, 251–254 (2016).
Bedzhov, I. & Zernicka-Goetz, M. Cell 156, 1032–1044 (2014).
Bryant, D. M. et al. Nature Cell Biol. 12, 1035–1045 (2010).
Blasky, A. J., Mangan, A. & Prekeris, R. Annu. Rev. Cell Dev. Biol. 31, 575–591 (2015).
Strilić, B. et al. Curr. Biol. 20, 2003–2009 (2010).
Buecker, C. et al. Cell Stem Cell 14, 838–853 (2014).
Fogarty, N. M. E. et al. Nature 550, 67–73 (2017).
Mangan, A. J. et al. Nature Commun. 7, 12426 (2016)

 Read the paper: Pluripotent state transitions coordinate morphogenesis in mouse and human embryos
Read the paper: Pluripotent state transitions coordinate morphogenesis in mouse and human embryos
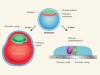 Implantation barrier overcome
Implantation barrier overcome
 Stem cells: Aspiring to naivety
Stem cells: Aspiring to naivety