Since COVID-19 emerged in January 2020, it has caused unprecedented disruption of clinical trials and ongoing patient care. Around 1,000 organizations have reported trial disruption, consistent with a reported ~80% decrease in new patients entering trials per site in April 2020 compared with April 2019 (see Related links). Of all active trials in ClinicalTrials.gov, 13% reported increases in trial duration in March–May 2020, compared with 9% over the same period in 2019 (Supplementary figure 1).
To protect patient safety and trial integrity, the pharmaceutical industry made strides to accelerate trial innovations such as digital tools and virtualization, with support from regulatory guidelines (see Related links). To help understand the recovery from COVID-19 disruption and the implications for the future conduct of clinical trials, we analysed data from ClinicalTrials.gov between January and July 2020 and surveyed 245 clinical trial investigators and study coordinators from around the world in May (see Supplementary Box 1 for details). Here, we present the results of this analysis and discuss the ongoing challenges for clinical trials, innovations to address them and actions to maximize impact.
Recovery of clinical trial activity
ClinicalTrials.gov data indicated decreases in new trial starts from January to May, and subsequent recovery in June and July to January levels (Fig. 1a,b). Similarly, after a surge in February and March, the number of trials suspended due to COVID-19 stabilized at ~1,200, and suspensions began to be lifted, with June being the first month with more suspensions lifted than newly imposed (Fig. 1c).

Fig. 1 | Resumption of global clinical trial activity. a | New trial starts recorded in ClinicalTrials.gov, including industry, government and investigator-sponsored trials. b | New trial starts in four therapeutic areas with the highest trial volume. c | Trials suspended in ClinicalTrials.gov explicitly citing COVID-19. d | 245 clinical trial investigators were surveyed on expectation of timing to trial restart between 8–18 May 2020. The countries most represented were the US (104), UK (33), Italy (19), Germany (17), Spain (16), France (12). See Supplementary Box 1 for details.
Our clinical trial investigator survey was conducted around the beginning of this recovery (8–18 May), when only 39% of active trials were still continuing as planned. However, 73% of investigators (65% in the US; 76% in the EU) already believed that the crisis peak had passed and 80% expected paused trials to restart within 2 months (Fig. 1d, Supplementary figure 1).
Innovation in trial operations
COVID-19 altered the conduct of ongoing trials, with investigators reporting 57% of patient interactions and 79% of interactions between sponsors and contract research organizations (CROs) taking place remotely (Fig. 2a). As in-person trial visits decreased, both industry and regulators anticipated the risk of resulting data loss. Regulators issued guidance on statistical mitigation approaches, while sponsors applied telemedicine, remote patient monitoring and EMR tools to replace in-person assessments and limit data gaps (see Related links).
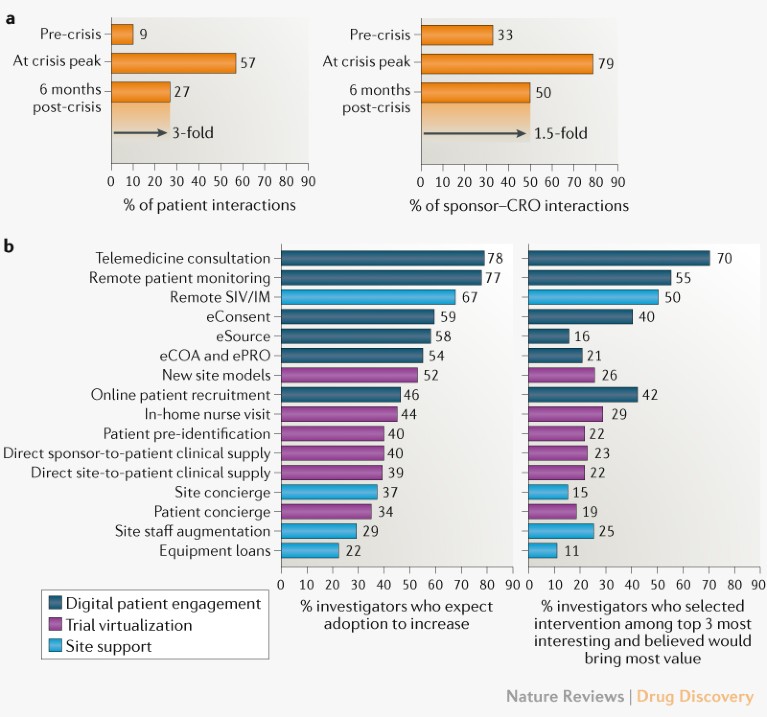
Fig. 2 | Remote engagement and trial digitization expected to persist post-crisis. a | A total of 245 clinical trial investigators were surveyed on the percentage of patient and sponsor–contract research organization (CRO) interactions taking place remotely before the crisis and their expectations for crisis peak and the future. b | Responses from investigators when asked whether each of 16 trial interventions presented in randomized order would increase in adoption post-crisis, and to select up to three interventions that they were most interested in and believed would bring most value. COA, clinical outcome assessment; IM, investigator meeting; PRO, patient-reported outcome; SIV, site initiation visit. See Supplementary Box 1 for details.
There are indications that such innovation adoption could be lasting. Investigators expected remote patient and sponsor–CRO interactions to persist at higher levels than pre-crisis (Fig. 2a, Supplementary figure 2). Among the investigators, 46–78% expected the adoption of digital patient engagement technologies to increase post-crisis. As patient engagement norms change, 34–52% expected trial decentralization approaches such as new site models, online recruitment, home nursing and direct-to-patient trial supply to increase (Fig. 2b, Supplementary figure 3)
These types of innovation had previously seen mixed adoption (Supplementary figure 3), and in some cases remain less tested. For example, remote monitoring had been named the least effective among five patient engagement initiatives in a 2016 survey, and as recently as November 2019, fewer than 40% of companies expected virtual trials to be a major component of their portfolio (see Related links).
Challenges for clinical trials
As clinical activity recovers, sponsors and other stakeholders are facing challenges on existing and new fronts.
Competition. With hundreds of COVID-19 vaccine and therapeutic trials launched each month in recent times and >1,000 suspended trials now resuming (Fig. 1c), sponsors face stiff competition for site, investigator and regulator bandwidth, requiring advance planning, active local monitoring, flexibility, and close partnership with sites and patients to sustain timelines.
Technology. Remote trial approaches were relatively uncommon before the crisis (Supplementary figure 3) and may still be fragmented. Scaling innovations will require improved data infrastructure for sponsors and sites. Moreover, in future the industry may begin to align around a finite set of common digital tools, forcing adopters and providers of alternative technologies to pivot.
Data collection. New data collection methods (Fig. 2b) are not yet commonplace; for example, eCOA/ePRO and eSource were only used by 48% and 14% of sponsors respectively in 2018 (Ther. Innov. Reg. Sci. 53, 71–80, 2019). These approaches may still be subject to data gaps, noise and biases, requiring rigorous validation and standardization before they can substitute for existing methods.
Sites of care. As trials move towards the home or online, the site operating model may evolve, potentially introducing new physicians to clinical research and supporting growth of smaller sites. Notably, 52% of investigators expected growth of new site models; for example, pharmacy, clinic or pop-up (Fig. 2b).
People. Roles and responsibilities for those involved in conducting clinical trials are likely to change; for example, site staff shifting towards providing or overseeing mobile healthcare, or digital records facilitating reduction in manual data entry and verification. Clinical researchers may require new capabilities, and at the same time be freed up to focus on more specialist work.
Actions to address the challenges
The challenges that COVID-19 poses to running clinical trials may persist until effective vaccines become widely available, and approaches used to address them are expected to become more widely used once the crisis is over (Fig. 2a). Stakeholders may therefore consider a long-term, holistic evaluation of trial design and conduct to adapt over the coming months and years. This could begin with five actions:
• Apply a design mindset to the personal experiences of patients and investigators, optimizing the unfamiliar virtual experience and learning from real-time feedback.
• Experiment and iterate in an evolving trial landscape, learning quickly from pilots before scaling up. Use this opportunity to not just address the immediate crisis, but innovate broadly across the planned trial portfolio.
• Cultivate broad partner ecosystems, and participate in technology, data and regulatory standardization to accelerate innovation and reduce adoption burden on investigators and sites.
• Examine the broader evidence generation toolkit, and consider approaches such as real-world evidence arms or digital prospective registries to supplement traditional, site-based randomized clinical trials.
• Grow digital fluency and invest in change management, enabling users to embrace new tools and apply them in the right contexts.
COVID-19 has demonstrated that sponsors and investigators can achieve in weeks what was previously thought to require months or years, and highlighted the humanitarian importance of efficient, robust and definitive clinical research. The industry’s actions over the next 1–2 years may determine fundamental pivots for how medicines are developed, giving each stakeholder an opportunity not only to adapt but to shape the future clinical trial paradigm.
