The COVID-19 pandemic is disrupting clinical research in much of the world. To understand how the crisis is affecting the management of ongoing oncology clinical trials and the planning of future trials, we used a combination of surveys and interviews of oncology clinical investigators globally and analyses of data from IQVIA and ClinicalTrials.gov on oncology clinical trials to investigate challenges, risks and contingencies. Here, we present data and insights from this investigation in the hope that they could be useful to those involved in oncology clinical trials.
Challenges for oncology clinical trials
Between 23 March and 3 April 2020, the Cancer Research Institute (CRI) and IQVIA surveyed a group of 36 investigators who are conducting clinical trials for cancer at institutions around the world. Separately, IQVIA conducted an internal analysis of a subset of its oncology clinical trials (n > 200) to identify major risks affecting the trials (see Supplementary Box 1 for details of the survey and data analysis).
Trial considerations. The survey results indicate that patient enrolment in active oncology clinical trials was negatively affected at the time of the survey, especially in the United States and Europe (Fig. 1a), with only 20% or 14% of the institutions continuing to enrol patients at the usual rate in these two regions, respectively (Fig. 1a). For institutions engaged in trials that were continuing, but with lower enrolment rates, patient care (as reflected by a comparison between in-patient and outpatient settings) was a key factor, with 9 out of 13 respondents identifying it as one of the top three considerations causing the most difficulty for patient enrolment in ongoing trials (Fig. 1b). The type of cancer therapy, including route of administration, was another key consideration, and the survey also revealed concerns about patient safety and a potential lack of research staff and resources. Interviews with investigators emphasized that a risk–benefit analysis for patients in ongoing trials was the fundamental consideration, regardless of the design or complexity of the trials (see Supplementary Box 1).
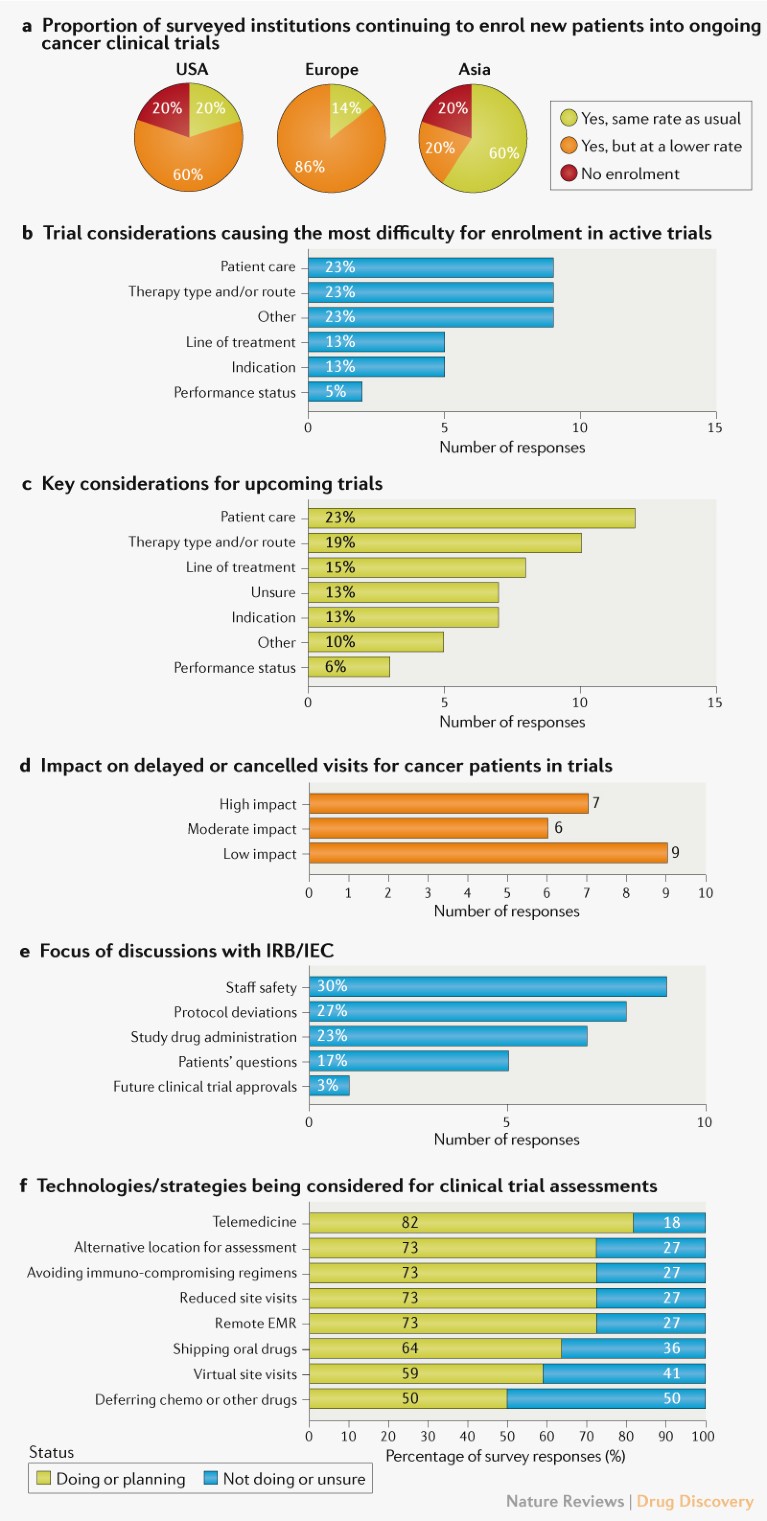
Fig. 1 | Impact of the COVID-19 pandemic on oncology clinical trials. a | Impact on patient enrolment in active oncology clinical trials, based on survey responses from 22 investigators leading trials in the United States (10), Europe (7) and Asia (5). b | Factors affecting enrolment for ongoing trials highlighted by 13 investigators, who selected their top three considerations. Percentages reflect responses within each category out of the total of 39 responses. c | Key considerations for future trials highlighted by 22 investigators. Percentages reflect responses within each category out of the total of 52 responses. d | Impact on patient visits based on responses from 22 investigators. e | Topics discussed with an institutional review board (IRB) or independent ethics committee (IEC). Percentages are out of the total of 30 responses from 11 investigators. f | Comparison of institutional strategies for clinical trial assessments and quality monitoring as a result of COVID-19. For each strategy, the percentage of respondents aware of current or future implementation and unaware of such measures at their institution are shown, based on 198 responses from 22 investigators. See Supplementary Box 1 for details. EMR, electronic medical records.
With regard to starting new trials, similar trends were observed in the survey responses, with patient care and cancer therapy type and route of administration again on top of the list of considerations (Fig. 1c). In follow-up interviews, therapy types requiring intravenous administration concerned the majority of investigators compared with oral therapies, as the latter could potentially be shipped to patients’ homes for self-administration.
Regulatory and operational considerations. As medical infrastructure became increasingly overwhelmed with COVID-19-related illnesses, we assessed how investigators were dealing with new regulatory and operational hurdles. Nearly 60% of investigators reported that the COVID-19 pandemic had ‘moderate’ or ‘high’ impact on patient visits (delayed or cancelled) (Fig. 1d). The majority (~80%) of the respondents anticipated that protocol deviations would cause unresolved queries, such as incomplete patient visit data.
Many of the investigators reported engaging with their ethics committees. The main focus areas of these committee engagements were the safety of their medical staff, deviations from existing protocols and administration of investigational drugs (Fig. 1e).
In order to mitigate operational challenges and ensure patient safety, investigators reported adopting or planning to adopt technology-based interventions aimed at reducing on-site monitoring visits and in-person patient visits to minimize potential viral exposure and spread, including telemedicine, remote electronic medical record access for monitors and virtual monitoring of data and study documentation (Fig. 1f). Other reported response strategies included shipment of oral drugs directly to patients and avoiding immunosuppressive treatment regimens (Fig. 1f).
Operational risks for ongoing trials. The separate analysis of a subset of IQVIA-run oncology clinical trials (n > 200) indicated milestone delays as the most commonly reported risk due to the pandemic (Fig. 2a). These predicted milestone delays will probably be caused by delays to site activation, enrolment, patient visits and/or data collection and cleaning.
Trial suspensions. Additional analysis of data from ClinicalTrials.gov indicates that over 200 interventional oncology studies were suspended in March and April owing to COVID-19 (Fig. 2b). A dynamic dashboard for the analysis is provided (see Related links).
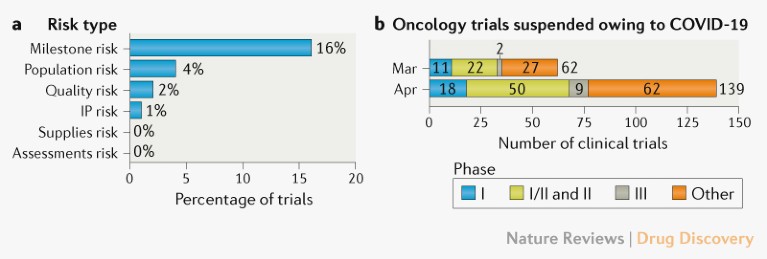
Fig. 2 | Risks and suspensions for ongoing oncology clinical trials. a | Operational risks, based on an analysis conducted by IQVIA of a subset of its oncology trials (n > 200). b | Number of interventional oncology trials suspended in March and April owing to COVID-19. Data extracted from ClinicalTrials.gov on 12 May 2020. ‘Other’ includes phase IV trials and trials for which the phase is not stated. See Supplementary Box 1 for details. IP, intellectual property.
Discussion and outlook
The main focus of our effort is to provide an initial assessment of the impact of the COVID-19 pandemic on the current landscape of clinical trials for cancer around the world. Although the data are limited by the sample size, they indicate that patient enrolment in active clinical trials for cancer therapies was severely affected by the COVID-19 pandemic during the survey assessment period. The situation is likely to be highly dynamic and regional, however, with less severe impacts in some cases. In this respect, the data indicate that enrolment in Asia was less affected at the time of the survey compared with Europe and the United States. This could be a result of the recent decline in the number of new cases in countries in the region. Indeed, all of the four clinical investigators interviewed in China, including one in Wuhan, reported that the clinical research infrastructure had either already returned to pre-pandemic levels, or was expected to return to full functionality by the end of April. By contrast, US-based investigators interviewed during our survey expected it would be 3–6 months before clinical research programs could be fully operational again. Some European investigators were reluctant to predict timelines for a return to normal operations as many regions were still observing a rise in the number of positive cases. Furthermore, owing to a lack of widespread screening and the possible prevalence of asymptomatic cases, investigators drew parallels from previous influenza epidemics and voiced concerns regarding the potential emergence of secondary waves of COVID-19.
The data from our investigation are consistent with a recent survey of US clinical investigators conducted by the American Society of Clinical Oncology and statements elsewhere from trial sponsors that have highlighted the risks of missing or delayed data collection from ongoing trials, and the challenges of initiating new clinical trials owing to the difficulties for patient enrolment (see Related links). Such statements also highlight the value of telemedicine, as indicated by our survey responses, as well as by investigators in the follow-up interviews we conducted (see Supplementary Box 1). Indeed, our findings emphasize the importance of getting telemedicine running smoothly in advance to allow the transition in patient care to be as seamless as possible as institutions become increasingly affected by COVID-19-related challenges.
Looking to the future, interviews also highlighted the possibility that the COVID-19 pandemic could have an enduring impact on clinical research and the practice of medicine in the future, with technological solutions such as telemedicine being adopted with increasing frequency even after the crisis. We believe additional future surveys including an increased number of investigators and regions as well as updated analyses of operational performance metrics are warranted to gain greater insight into the impact of the pandemic and into solutions that could help in mitigating its impact on clinical trials.
