Abstract
Polycomb group genes play crucial roles in the maintenance of the transcriptionally silenced state of genes for proper cell differentiation in animals and plants. While components of the polycomb repressive complex2 (PRC2) are evolutionarily conserved and their functions are extensively studied in plants, PRC1 differs considerably between animals and plants, and its functions in plants are as yet not well described. Previous studies have identified the Arabidopsis AtRING1a and AtRING1b as homologues of the animal PRC1 subunit RING1. Here, we show that the Atring1a Atring1b double mutant exhibits derepression of embryonic traits during vegetative growth. Accordingly, several key regulatory genes involved in embryogenesis and stem cell activity are ectopically expressed in the mutant. Furthermore, we show that the mutant phenotypes and increased expression of regulatory genes are enhanced by the PRC2 mutant clf. Finally, we show that three homologues of the animal PRC1-subunit ring-finger protein BMI1, AtBMI1a, AtBMI1b and AtBMI1c, can bind with AtRING1a or AtRING1b, and in addition, AtBMI1c can bind with LHP1. The Atbmi1a Atbmi1b double mutant shows derepression of embryonic traits similar to that of the Atring1a Atring1b double mutant. Interestingly, expression levels of AtBMI1a, AtBMI1b and AtBMI1c are elevated in the Atring1a Atring1b mutant and those of AtBMI1c, AtRING1a and AtRING1b are elevated in the Atbmi1a Atbmi1b mutant, suggesting a self-regulatory feedback mechanism. Taken together, our results illuminate crucial functions of the PRC1-like ring-finger components in stable repression of embryonic traits and regulatory genes for proper somatic growth.
Similar content being viewed by others
Introduction
Polycomb group (PcG) proteins exist in multiprotein complexes; the best characterized of these are known as polycomb repressive complex1 (PRC1) and PRC2 in Drosophila and mammals. PRC2, via its catalytic subunit E(z), methylates histone H3 lysine 27 (H3K27), resulting in trimethyl-H3K27 (H3K27me3), and PRC1, via its chromodomain-containing subunit polycomb (Pc), binds H3K27me3 resulting in a stable silencing chromatin state (reviewed in Schuettengruber et al.1). In the model plant Arabidopsis thaliana, PRC2 subunits are evolutionarily conserved, thus MEDEA (MEA), CURLY LEAF (CLF) and SWINGER (SWN) are E(z) homologues; EMBRYONIC FLOWER2 (EMF2), FERTILISATION INDEPENDENT SEED2 (FIS2) and VERNALIZATION2 (VRN2) are Su(Z)12 homologues; FERTILIZATION INDEPENDENT ENDOSPERM (FIE) is the unique ESC homologue; and MULTICOPY SUPRESSOR OF IRA1-5 (MSI1-5) are RbAp46/48 homologues, but only MSI1 has been demonstrated as part of the PRC2 complexes so far (reviewed in Pien and Grossniklaus2 and Alvarez-Venegaz 3). Homologues of PRC2 complexes are involved in many aspects of plant development, including the repression of flowering during vegetative development, the suppression of endosperm development in the absence of fertilization, and the repression of stem cell pluripotency for cell differentiation (reviewed in Pien and Grossniklaus2, Alvarez-Venegaz 3 and Shen and Xu 4). In comparison, much less is known about PRC1 function in plants. Arabidopsis does not contain any homologue of Pc. Nevertheless, the chromodomain-containing protein LIKE HETEROCHROMOTIN PROTEIN1 (LHP1) binds H3K27me3 and thus can play a Pc-analogous function in Arabidopsis 5, 6. In addition to Pc, the animal PRC1 core complex contains ring-finger proteins RING1A, RING1B and BMI1 1. Arabidopsis has two RING1-homologues, AtRING1a and AtRING1b, which bind to LHP1 and together are involved in repression of Class I KNOX genes for the maintenance of proper shoot stem cell activity 7. Arabidopsis also contains three genes encoding ring-finger proteins that show closer homology to BMI1 7, 8, which we name accordingly hereinafter as AtBMI1a (At1g06770), AtBMI1b (At2g30580) and AtBMI1c (At3g23060). Qin et al. 9 reported that AtBMI1a (also named DRIP1) and AtBMI1b (also named DRIP2) are involved in ubiquitin-dependent proteasomal degradation of the transcriptional regulator DREB2A, which is involved in drought stress response. The possible PRC1-like function of AtBMI1a, AtBMI1b and AtBMI1c is unexamined to date. In this study, we show that the double mutant Atring1a Atring1b, as well as Atbmi1a Atbmi1b, exhibits derepression of embryonic traits in somatic plant tissues, and that AtRING1a and AtRING1b bind AtBMI1a, AtBMI1b and AtBMI1c. We propose that AtRING1 and AtBMI1 proteins have non-redundant functions within a PRC1-like complex, which is crucial for the maintenance of differentiated somatic cell fate during post-embryonic plant development.
Results
AtRING1a::AtRING1a-GUS is expressed at high levels in vegetative tissues containing actively proliferating cells
Previous RT-PCR analysis showed that AtRING1a and AtRING1b are ubiquitously expressed in several examined plant organs including roots, stems and leaves 7. In order to further investigate AtRING1a expression, we constructed AtRING1a::AtRING1a-GUS, which contains the AtRING1a promoter (−1 359 bp) and entire coding region (+4 356 bp; including all introns and exons) fused in frame with the β-glucuronidase reporter gene GUS. Transgenic plants containing AtRING1a::AtRING1a-GUS were investigated for GUS activity by histochemical staining.
While GUS activity was undetectable in imbibed seeds (Figure 1A), GUS staining was clearly visible in the root apical meristem (RAM) as early as 1 day after seed stratification (DAS) (Figure 1B) and in the RAM and shoot apical meristem (SAM) starting from 2 DAS (Figure 1C). This appearance of GUS staining over time correlates relatively well with the previously established time transition from quiescent to active cell proliferation in RAM and SAM during seed germination 10. At later growth stages, GUS staining was observed in young leaves and vasculature of older leaves (Figure 1D), where cell proliferation is active; in the junction zone between the root and shoot (Figure 1E), where adventitious roots initiate; in lateral root primordium (Figure 1F); and at all stages of growth in RAM (Figure 1G) and SAM (Figure 1D). Together, these results indicate that AtRING1a::AtRING1a-GUS expression is closely associated with actively proliferating cells.
Spatiotemporal expression of AtRING1a::AtRING1a-GUS in transgenic Arabidopsis plants. (A) Imbibed seeds at 4 °C for 2 days. (B) Seedlings grown on culture medium at 1 day after stratification (DAS). (C) Seedling at 2 DAS. (D , E , F , G) Aerial part, shoot-root junction, lateral root primordium region and primary root tip, respectively, from a 1-month-old seedling. Samples were incubated in GUS staining solutions for 2 h (A-D) or for 16 h (E-G). Note blue staining that indicates presence of GUS activity. Scale bar = 250 μm (A-C), 2 mm (D) and 100 μm (E-G).
The double mutant Atring1a Atring1b displays derepression of embryonic traits
Our previous work demonstrated SAM defects in the double mutant Atring1a Atring1b 7; however, the cell fate of abnormal tissue generation in the mutant had not been investigated. The observation that AtRING1a::AtRING1a-GUS expression is associated with cell proliferation early after the break of seed dormancy prompted us to examine whether AtRING1a and AtRING1b play a role in the maintenance of somatic cell fate in post-embryonic plant development. We investigated the Atring1a Atring1b-mutant phenotype of plants grown on in vitro culture medium. We used Fat Red dye staining to reveal the presence of neutral lipids, which accumulate during seed maturation and disappear during vegetative growth, thus serving as an indicator of embryonic traits 11. As expected, no red staining was detectable in 1-month-old wild-type plants (Figure 2A). Interestingly, the Atring1a Atring1b mutant showed red staining of SAM and RAM zones in growth-arrested plants (Figure 2B). Staining was also observed in ectopic calli formed on cotyledons, leaves or from the SAM zone of mutant plants (Figure 2B and 2C). The mutant embryonic calli (EC) proliferated and could produce leaf-like structures (Figure 2D and 2E). The differentiated leaf-like structures largely do not display embryonic traits; red staining is visible only on undifferentiated parts of EC (Figure 2D and 2E). In addition to red staining, the distal end of primary root was swollen and greenish in appearance. A similar root phenotype was previously described in the pickle (pkl) mutant 12; thus, we hereinafter use pickle-root to describe the Atring1a Atring1b-mutant root phenotype. Starch accumulation is another seed trait; while only a few columella cells contain starch granules in wild-type roots, the pickle-roots from Atring1a Atring1b showed additional starch granule accumulation at the distal end (Figure 2F), further revealing the abnormal presence of embryonic traits in somatic cells of the Atring1a Atring1b mutant. The pickle-root tip is thicker, contains more cell layers, and is arrested in elongation (Figure 2G). The EC and pickle-root phenotypes are not fully co-segregated in the Atring1a Atring1b mutant. The penetrance of EC and pickle-root was roughly 17% and 50%, respectively, and together account for ∼58% of Atring1a Atring1b-mutant plants showing derepression of embryonic traits (Figure 2H).
Phenotypes of the Atring1a Atring1b-mutant plants. (A) Wild-type plant (1-month old) stained by Fat Red. (B , C) Atring1a Atring1b plants (1-month old) stained by Fat Red. Note embryonic calli (EC) and pickle-root segments that display triacylglycerol accumulation stained in red. (D , E) Atring1a Atring1b plants (2-month old) stained by Fat Red. Note growth of leaf-like structures from EC. (F) Iodo-starch staining of wild-type (left panel) and Atring1a Atring1b (right panel) roots. Note starch granules (in dark color) that appear in columella cells at tip of both wild-type and mutant roots, and in pickle-root cells at distal zone of mutant roots only. (G) Close-up of root tip from wild-type (upper panel) and Atring1a Atring1b (lower panel). (H) Quantitative analysis showing penetrance of EC and pickle-root phenotypes in 1-month-old Atring1a Atring1b plants. Total shows percentage of plants exhibiting EC and/or pickle-root phenotypes. A sum of 578 plants were scored. Error bars represent standard deviation from triplicate repeats. Scale bar = 1 mm (A-C), 5 mm (D), 1 mm (E), 100 μm (F) and 50 μm (G).
AtRING1a and AtRING1b are required for stable repression of key regulatory genes involved in embryogenesis and stem cell activity
To investigate the molecular events underlying derepression of embryonic traits in Atring1a Atring1b-mutant plants, we analyzed expression levels of selected key regulatory genes involved in stem cell activity and embryogenesis (Figure 3A). Consistent with our previous findings 7, the key SAM-regulatory genes (STM, BP/KNAT1, KNAT2 and KNAT6) encoding Class I KNOX transcription factors are upregulated by 2- to 6-fold in the mutant. The NAC-domain transcription factor genes CUC1, CUC2 and CUC3, which are required for organ boundary establishment and SAM initiation 13, are upregulated by 3- to 15-fold in the double mutant (Figure 3A). While the homeodomain transcription factor gene WUS and its homologue WOX2, which are essential for SAM organizing center activity and apical embryo-axis cell fate 14, 15, are only slightly upregulated, WOX5 and WOX8, which are crucial for RAM function and basal embryo-axis cell fate termination 15, are upregulated by more than 5-fold in the mutant (Figure 3A). The embryonic competence-enhanced factor gene AGL15 16 is upregulated by more than 15-fold, whereas expression of the somatic embryogenesis receptor-like kinase genes SERK1 and SERK2 17 is unaffected in the mutant (Figure 3A). Drastic upregulation of expression (from 18- to more than 360-fold) was observed for several key embryonic regulatory genes (Figure 3A), including BBM encoding an AP2/ERF transcription factor 18, LEC1 encoding a CCAAT-binding transcription factor 19, as well as LEC2, FUS3 and ABI3 encoding B3 domain factors 20, 21, 22. It is known that the phytohormone auxin plays an important role in embryogenesis and somatic embryo formation 23. We detected a 3- to 4-fold upregulation of PIN1 and PIN2 in the mutant, but neither PIN4 nor PIN7 expression was affected, all from a gene family encoding polar auxin transporters 24 (Figure 3A). Taken together, our results show that some but not all stem cell and embryonic regulatory genes are ectopically derepressed in Atring1a Atring1b.
Expression analysis of embryonic and stem cell regulatory genes in Atring1a Atring1b. (A) Quantitative RT-PCR analysis of gene expression in 2-week-old seedlings. Relative expression levels are shown as induction fold in Atring1a Atring1b compared with wild-type (set as 1). Error bars represent standard deviation from triplicate repeats. (B) Spatial expression of the embryonic regulatory gene reporter ABI3::GUS in wild-type (upper panel) and Atring1a Atring1b (lower panel) plants. (C) Spatial expression of the stem cell regulatory gene reporter BP::GUS in wild-type (left panel) and Atring1a Atring1b (right panel) plants. (D) Spatial expression of the cell division reporter gene CYCB1::GUS in wild-type (left panel) and Atring1a Atring1b (right panel) plants. Scale bar = 1 mm.
To further investigate the association of gene expression and the mutant phenotype, we introduced by genetic cross the ABI3::GUS 25 and CYCB1::GUS 26 reporter genes into the Atring1a Atring1b mutant. While ABI3::GUS expression is restricted to the SAM zone in wild-type plants (left panel in Figure 3B), in the Atring1a Atring1b-mutant plants, GUS staining is, in addition, clearly visible in some regions of cotyledons (right panel in Figure 3B) and leaves (data not shown). This ectopic expression pattern of ABI3::GUS is very similar to that of BP::GUS (Figure 3C; Xu and Shen 7), indicating that both genes are ectopically derepressed in somatic cells of the Atring1a Atring1b mutant. The reporter CYCB1::GUS is expressed during late G2 and M phases of the cell cycle, thus providing a marker of cell division 26. In wild-type seedlings, GUS activity is detected in SAM and young leaves, in junction between shoot and root, in lateral root primordium and the root tip (left panel in Figure 3D). In Atring1a Atring1b seedling, GUS activity is additionally visible in some regions in cotyledons and leaves, and in pickle-root vasculature (right panel in Figure 3D). Together, the observed reporter gene expression patterns suggest that somatic embryogenesis and stem cell activities are associated with derepression of regulatory genes in the Atring1a Atring1b mutant.
Derepression of embryonic traits of the Atring1a Atring1b mutant is inhibited by NPA treatment, but enhanced by clf or as1 mutation
To investigate whether auxin distribution plays a role in the establishment of the Atring1a Atring1b-mutant phenotype, we grew plants on culture medium in the presence of the auxin transport inhibitor 1-naphthylphthalamic acid (NPA). As shown in Figure 4A, NPA effectively reduced both EC and pickle-root penetrance in the Atring1a Atring1b mutant. This is consistent with the well-established importance of the auxin gradient in embryogenesis and somatic embryo formation 23, and further indicates that a similar auxin function is necessary for expression of ectopic stem cell fate and embryonic traits in the Atring1a Atring1b mutant.
The auxin transporter inhibitor NPA inhibits and clf or as1 enhances Atring1a Atring1b-mutant phenotypes. (A) Effect of NPA on the penetrance of embryonic calli (EC), pickle-root and total phenotypes in 1-month-old Atring1a Atring1b mutants. The number of scored plants is indicated for each growth condition within brackets. Error bars represent standard deviation from triplicate repeats. (B) Comparison of the penetrance of EC, pickle-root and total phenotypes in 1-month-old plants of the Atring1a Atring1b, Atring1a Atring1b clf and Atring1a Atring1b as1 mutants. The number of scored plants is indicated for each mutant within brackets. Error bars represent standard deviation from triplicate repeats. (C) Phenotype of 1-month-old Atring1a Atring1b clf (upper panel) and Atring1a Atring1b as1 (lower panel) plants stained by Fat Red. Scale bar = 1 mm. (D) Quantitative RT-PCR analysis of gene expression in 1-month-old plants of the Atring1a Atring1b, Atring1a Atring1b clf and Atring1a Atring1b as1 mutants. Relative expression levels are shown as induction fold in the mutant compared with wild-type (set as 1). Error bars represent standard deviation from triplicate repeats.
PKL and its homologue PKR2 have been shown to promote H3K27me3 and activate the PRC2 genes CLF, SWN and EMF2 11, 27. Consistently, the clf swn and emf2 vrn2 mutants also exhibit ectopic cell dedifferentiation and somatic embryogenesis 28. Investigation of the Atring1a Atring1b clf triple mutant 7 revealed that the EC and pickle-root penetrance of Atring1a Atring1b were greatly and slightly enhanced by clf, respectively (Figure 4B and 4C). ASYMMETRIC LEAVES1 (AS1), encoding a MYB-domain transcription factor, is involved in repression of the Class I KNOX genes BP, KNAT2 and KNAT6 to promote leaf formation 29, and pkl has been shown to enhance as1 effects 30. To examine the genetic interaction between as1 and Atring1a Atring1b, we obtained and studied the Atring1a Atring1b as1 triple mutant. We found that as1 greatly enhanced EC phenotype and frequency, but only slightly the pickle-root penetrance, in the Atring1a Atring1b mutant (Figure 4B and 4C). Consistent with their enhanced phenotype, both the Atring1a Atring1b clf and Atring1a Atring1b as1 triple mutants showed elevated derepression of embryonic and stem cell regulatory genes (Figure 4D). Taken together, these genetic data suggest that several complexes in conjunction with PRC2 and PRC1-like components act in repression of embryonic and stem cell regulatory genes to prevent dedifferentiation of somatic cells.
Identification of BMI1 homologues as putative PRC1-like components
To investigate the Arabidopsis BMI1-homologues as potential PRC1-like components, we first examined their physical interaction with LHP1, AtRING1a and AtRING1b in yeast two-hybrid assays. We found that AtBMI1a, AtBMI1b and AtBMI1c could bind with AtRING1a or AtRING1b, but only AtBMI1c could bind with LHP1 (Figure 5A). Consistent with previous reports 7, 31, we found that AtRING1a binds LHP1 and that LHP1 binds to itself (Figure 5A). To further confirm protein-protein interactions, we produced glutathione S-transferase (GST) fusion proteins, GST-AtBMI1a, GST-AtBMI1b and GST-AtBMI1c, and used them in pulldown assays. The assays were performed on total protein extract prepared from transgenic plants expressing FLAG-AtRING1a 7. As shown in Figure 5B, the anti-FLAG antibody detected FLAG-AtRING1a in input as well as in pulldown fractions by GST-AtBMI1a, GST-AtBMI1b or GST-AtBMI1c, but not by GST alone. Similarly, our previous study showed that GST-LHP1 can pulldown FLAG-AtRING1a 7. Taken together, these observed physical interactions support that AtBMI1, AtRING1 and LHP1 could form protein complexes.
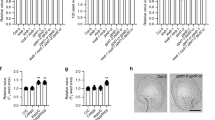
Examination of Arabidopsis BMI1 homologs as putative PRC1-like components. (A) Yeast two-hybrid assay for protein-protein interaction between putative PRC1-like components. Dilution (×10) series of yeast cells coexpressing the indicated proteins from the pGBKT7 and pGADT7 vectors were plated onto SD-LT or selective medium SD-LTH+3AT. Growth of yeast cells on SD-LTH+3AT plate indicates positive interaction. (B) Pull-down assay. Total protein extract from 15-day-old seedlings expressing FLAG-AtRING1a was subdivided into four and incubated with GST-AtBMI1a-, GST-AtBMI1b-, GST-AtBMI1c- or GST-coated beads. The pull-down fractions were analyzed by western blotting with antibodies against the FLAG epitope. Arrowhead indicates band corresponding to the size of FLAG-AtRING1a. Asterisk indicates band of an unknown protein, present in input but absent from pull-down fractions. (C-E) Similar to the Atring1a Atring1b mutant, the Atbmi1a Atbmi1b mutant shows Fat Red staining in arrested SAM (C), in somatic embryonic calli (D) and in pickle-root regions (E). Scale bar = 1 mm. (F) Penetrance of embryonic calli (EC), pickle-root and total phenotypes in 1-month-old Atbmi1a Atbmi1b plants. A sum of 263 plants was scored. Error bars represent standard deviation from triplicate repeats. (G) Quantitative RT-PCR analysis of gene expression in 2-week-old seedlings. Relative expression levels are shown as induction fold in Atbmi1a Atbmi1b compared with wild-type (set as 1). Error bars represent standard deviation from triplicate repeats.
Although all three proteins AtBMI1a, AtBMI1b and AtBMI1c show sequence homologies with mammalian BMI1 (Supplementary information, Figure S1), they are differently related. AtBMI1a and AtBMI1b amino acid sequences show 68% identity and 80% similarity to each other, but only show, respectively, 44% and 50% identity with AtBMI1c. It was previously reported that simultaneous loss-of-function of AtBMI1a/DRIP1 and AtBMI1b/DRIP2 delays plant development and growth 9. We obtained the corresponding T-DNA insertion mutants, Atbmi1a (previously named drip1-1 in Qin et al. 9) and Atbmi1b (previously named drip2-1 in Qin et al. 9), and we generated the double mutant Atbmi1a Atbmi1b. In support of a PRC1-like function for AtBMI1a and AtBMI1b, we found that the Atbmi1a Atbmi1b double mutant (but not the single mutants) showed EC and pickle-root phenotypes (Figure 5C-E and Supplementary information, Figure S2), which are largely similar to those of the Atring1a Atring1b mutant. The penetrance of EC and pickle-root was nearly 18% and 8%, respectively, and together account for ∼21% of Atbmi1a Atbmi1b-mutant plants showing derepression of embryonic traits (Figure 5F). Consistent with derepression of embryonic traits, upregulation of expression in Atbmi1a Atbmi1b was detected to varying extents for CUC1, WOX5, WOX8, AGL15, PIN2, BBM, LEC1, LEC2, FUS3 and ABI3 (Figure 5G).
Taken together, our data suggest that AtRING1 and AtBMI1 proteins play non-redundant functions within a common PRC1-like complex involved in repression of regulatory gene transcription and embryonic traits during post-embryonic plant growth.
Gene expression analysis reveals a reciprocal repression between AtRING1 and AtBMI1 genes
The presence of multiple genes encoding ring-finger components of the PRC1-like complex is intriguing. To gain insight into their regulation, we investigated expression of AtRING1a, AtRING1b, AtBMI1a, AtBMI1b, AtBMI1c and several others genes involved in repression of embryonic traits and stem cell activity in somatic cells. We found that AtBMI1c and, to a less degree, AtBMI1a and AtBMI1b were all upregulated in the Atring1a Atring1b mutant (Figure 6A), and that AtRING1a and AtRING1b, as well as AtBMI1c, were upregulated in the Atbmi1a Atbmi1b mutant (Figure 6B). PKR2 expression was also increased in the Atring1a Atring1b mutant and to a less degree in the Atbmi1a Atbmi1b mutant. Slightly decreased PKL expression was observed in the Atbmi1a Atbmi1b mutant and an increase of VAL1 expression was observed in the Atring1a Atring1b mutant. The remaining genes examined, including LHP1, CLF, SWN, EMF2 and VRN2, did not show expression changes in either double mutant (Figure 6). The observation of reciprocal repression of AtRING1a/AtRING1b and AtBMI1a/AtBMI1b suggests that these PRC1-like ring-finger genes are themselves repressed by the PRC1-like complex.
Quantitative RT-PCR analysis of gene expression in 2-week-old seedlings of the Atring1a Atring1b (A) and Atbmi1a Atbmi1b (B) mutants. Relative expression levels are shown as induction fold in the mutant compared with wild-type (set as 1). Error bars represent standard deviation from triplicate repeats.
Discussion
PRC1-like complexes in Arabidopsis
Currently, several lines of evidence support the presence of PRC1-like complexes in Arabidopsis. First, LHP1 binds H3K27me3 through its chromodomain and this binding is crucial for LHP1 function 5, 6, 32, supporting the hypothesis that LHP1 is functionally analogous to the animal PRC1 component Pc in Arabidopsis. Second, AtRING1a and AtRING1b, as well as AtBMI1a, AtBMI1b and AtBMI1c, show high sequence homology and similar domain organization to their animal homologues RING1 and BMI1 7, 8; both RING1 and BMI1 are core components of the PRC1 complex in animals. Third, physical interaction has been detected for LHP1 with AtRING1a or AtBMI1c; for AtRING1a with AtRING1b, AtBMI1a, AtBMI1b or AtBMI1c; and for AtRING1b with AtBMI1a, AtBMI1b or AtBMI1c (Xu and Shen 7; this study), supporting several possible combinations of multiprotein complexes. In another line of supporting evidence, GUS reporter expression analysis reveals that AtRING1a (this study), AtBMI1a/DRIP1 9 and LHP1 33 have similar expression patterns, with high expression levels found in plant tissues containing actively proliferating cells, supporting possible ensemble action of these genes. Finally, AtRING1a and AtRING1b share with LHP1 a common mechanism in repression of some PcG-target genes, which does not involve any change in H3K27me3 deposition 7 and is consistent with the known PRC1 function in stabilization of gene repression downstream of PRC2-mediated H3K27me3 deposition.
Among the five ring-finger genes, AtRING1a and AtRING1b show a redundant function, and AtBMI1a and AtBMI1b are also functionally redundant. Consistent with the view that AtRING1 and AtBMI1 play non-redundant roles within a PRC1-like complex, the Atring1a Atring1b and Atbmi1a Atbmi1b mutants show largely similar embryonic derepressive phenotypes. So far, the function of AtBMI1c has not yet been examined. Interestingly, AtBMI1c expression is upregulated in both the Atring1a Atring1b and Atbmi1a Atbmi1b mutants, AtRING1a and AtRING1b expression is upregulated in the Atbmi1a Atbmi1b mutant and AtBMI1a and AtBMI1b expression is upregulated in the Atring1a Atring1b mutant; thus, revealing that repression of the PRC1-like ring-finger genes is regulated by themselves. Distinct from the largely similar phenotypes observed between the Atring1a Atring1b and Atbmi1a Atbmi1b mutants, the lhp1 mutant has a relatively different phenotype 31, and lhp1 enhances the Atring1a Atring1b phenotype 7. This indicates that LHP1 might not be the only Arabidopsis protein recognizing H3K27me3, but that there are other LHP1-independent pathways acting in parallel for AtRING1 and AtBMI1 function. Future identification of novel components and further biochemical characterization of PRC1-like complexes will provide further insight into PcG-mediated gene silencing in plants.
PRC2 and PRC1-like complexes in repression of embryonic traits and stem cell activities in somatic cells
The Atring1a Atring1b and Atbmi1a Atbmi1b (drip1-1 drip2-1) mutants show pleiotropic phenotypes 7, 9. Our study focusing on these mutants during seedling growth reveals a crucial function of PRC1-like complexes in repression of embryogenesis and stem cell activities for proper vegetative growth. Plants displaying a severe mutant phenotype fail to form true leaves and display EC and/or pickle-root phenotypes, which could occur simultaneously within the same plant. EC formation could be observed on various regions of the plant, including the SAM region, cotyledons, leaves and roots, suggesting a general rather than organ-specific requirement of AtRING1a and AtRING1b, as well as AtBMI1a and AtBMI1b in embryonic trait repression. Pleiotropy and variable expressivity of mutant phenotypes among individual plants are not specific characteristics of Atring1a Atring1b and Atbmi1a Atbmi1b, but occur frequently in many chromatin modification or remodeling-defective mutants in plants. This variability could be associated with the high degree of plasticity of plant growth and development, which is essential for plants to cope with environmental changes. While the penetrance of EC is similar in the Atring1a Atring1b and Atbmi1a Atbmi1b mutants (17-18%), the penetrance of pickle-root phenotype in Atring1a Atring1b (∼50%) is significantly higher than that in Atbmi1a Atbmi1b (∼8%). It will be interesting in future experiments to investigate whether AtBMI1c has a redundant function with AtBMI1a and AtBMI1b in repression of embryonic traits in roots. The penetrance of pickle-root phenotype was previously reported to be low in pkl (< 10%) but enhanced in pkl pkr2 (32-40%, Aichinger et al. 11).
Consistent with the strongly derepressed embryonic traits, many regulatory genes involved in embryogenesis and stem cell maintenance are upregulated in the Atring1a Atring1b mutant. These include the key embryonic regulatory genes LEC1, LEC2, FUS3, ABI3 and BBM 18, 19, 20, 21, 22, the embryonic competence-enhanced gene AGL15 16, the key RAM-regulatory and basal embryo-axis cell fate genes WOX5 and WOX8 15, the key SAM-regulatory genes STM, BP, KNAT2 and KNAT6 7, the organ boundary regulatory genes CUC1, CUC2 and CUC3 13 and the auxin transporter genes PIN1 and PIN2 24. Remarkably, treatment with the auxin transporter inhibitor NPA can reduce the penetrance of embryonic traits in Atring1a Atring1b, indicating that the normal requirement of polar auxin gradient in embryogenesis 23 is maintained in ectopic embryonic trait development in the mutant. The LEC1, LEC2, FUS3, ABI3, BBM, AGL15, WOX5, WOX8, CUC1 and PIN2 genes were also found to be upregulated to varying extents in the Atbmi1a Atbmi1b mutant. Interestingly, the Class I KNOX genes (STM, BP, KNAT2 and KNAT6) were upregulated in Atring1a Atring1b, but barely changed in Atbmi1a Atbmi1b. This is consistent with the highly fasciated stem phenotype observed in Atring1a Atring1b 7 but not in Atbmi1a Atbmi1b (drip1-1 drip2-1, Qin et al. 9). Again, the difference between Atring1a Atring1b and Atbmi1a Atbmi1b might be explained by the existing AtBMI1c function. Alternatively, as AtBMI1a/DRIP1 and AtBMI1b/DRIP2 were shown to target the transcription factor DREB2A to proteasome degradation 9, the AtRING1 and AtBMI1 proteins might have additionally independent roles in modifying transcription factors to modulate gene transcription.
The LEC1, LEC2, FUS3 and ABI3 genes were also upregulated in pkl and more drastically in pkl pkr2 11, 27. The chromodomain/helicase/DNA-binding domain (CHD3) proteins PKL and PKR2 can activate several PRC2 component genes including CLF, SWN and EMF2, thus suggesting that derepression of embryonic traits and regulatory genes in pkl and pkl pkr2 is caused by reduced PRC2 activity 11, 27. Distinctively, the derepression of embryonic traits and regulatory genes in Atring1a Atring1b and Atbmi1a Atbmi1b is not associated with any detectable changes in expression of the PRC2 component genes. Upregulation of PKR2 was detected in both Atring1a Atring1b and Atbmi1a Atbmi1b, suggesting a feedback loop that could compensate reduced PRC1-like activity. Nevertheless, effectiveness of such a regulatory mechanism will need to be verified in future experiments. Both clf swn and emf2 vrn2 show ectopic formation of embryo-like structures 28, and at least in the case of clf swn the structures developing from above-ground organs exhibit Fat Red staining 11. Consistently, ectopic derepression of LEC1, FUS3 and STM was observed in clf swn 34, 35. Our triple mutant analysis revealed that clf enhances the Atring1a Atring1b-mutant phenotype and the derepression of LEC1, FUS3 and STM, as well as LEC2, AGL15, BP and KNAT6 (Figure 4). We also found that as1 has a similar function in enhancing the Atring1a Atring1b-mutant phenotype and the Atring1a Atring1b as1 triple mutant exhibits elevated derepression of embryonic and stem cell regulatory genes (Figure 4), suggesting that AS1 establishes leaf cell fate via a link with the PcG pathway. A previous study showed that the as1-mutant phenotype is enhanced by pkl, which is associated with elevated derepression of BP and KNAT2 30. Further experiments are necessary to better understand the molecular mechanisms underlying the link between AS1 and the PKL and PcG pathways.
In conclusion, this work together with previous studies, establishes the crucial function of PcG-mediated gene silencing mechanisms in the maintenance of stable repression of embryonic traits for post-embryonic plant growth and development. The strong somatic embryogenesis phenotypes associated with derepression of a large number of embryonic and stem cell regulatory genes, as observed in Atring1a Atring1b and in Atbmi1a Atbmi1b (drip1-1 drip2-1), reveal a central role of the PRC1-like ring-finger components, which is in agreement with the well-established function of animal PRC1, known to act downstream of PRC2 in maintenance (as opposed to initiation) of PcG-mediated gene silencing. Several pathways, including PRC2, PKL and AS1, likely work in conjunction with the PRC1-like complexes in stable repression of the embryonic program in somatic cells. Such a stable yet flexible repression system might be advantageous to cope with the highly frequent cell differentiation occurring during plant organogenesis and with the remarkable developmental plasticity of plants.
Materials and Methods
Plant materials and growth conditions
All Arabidopsis thaliana mutants were derived from the Columbia ecotype. Mutants as1-1, Atbmi1a/drip1-1 (WiscDsLox437G06) and Atbmi1b/drip2-1 (SALK_145041) were obtained from the Arabidopsis Biological Resource Center (ABRC, http://www.Arabidopsis.org). The Atring1a Atring1b double mutant and the Atring1a Atring1b clf triple mutant have been previously described 7. Other combined mutant and reporter gene lines were obtained by appropriate genetic crosses. For plant growth, seeds were surface sterilized (70% and 95% ethanol for 10 min) and plated on MS medium (MS salts, 1% sucrose (pH 5.8), 0.9% bactoagar). For NPA effect assay, the MS medium was supplemented with NPA (Sigma-Aldrich, http://www.sigmaaldrich.com) at the specified concentrations. After stratification at 4 °C for 2 days in the dark, plates were incubated in a growth chamber at 22 °C under a 16-h light/8-h dark photoperiod.
Plasmid construction and plant transformation
For AtRING1a::AtRING1a-GUS construction, the genomic DNA fragment containing the upstream promoter and the entire coding region of the AtRING1a gene (from −1 359 bp to +4 356 bp) was PCR-amplified from BAC K9L2 using specific primers (Supplementary information, Table S1). The DNA fragment was digested and cloned into pBI101 (Clontech, http://www.clontech.com) using SalI and BamHI. The GUS reporter gene was cloned in frame at the C-terminal end of AtRING1a (AtRING1a::AtRING1a-GUS). The binary vector was introduced into Agrobacterium tumefaciens GV3101, which was used to transform Arabidopsis plants by the floral-dip method 36.
GUS histochemical assays
For histochemical GUS activity assays, seedlings were submerged in 90% acetone for 30 min on ice, washed twice with 50mM sodium phosphate buffer (pH 7.2) for 15 min at room temperature and subsequently incubated in staining solution (0.1 M sodium phosphate buffer (pH 7.2), 0.5 mM Fe(CN)2, 0.5 mM Fe(CN)3, 0.1% Tween-20 and 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide) at 37 °C for 1-12 h, depending on the activity of each reporter gene construct. Seedlings were cleared by incubation overnight in 70% ethanol at 4 °C.
Fat Red staining
Whole seedlings were stained overnight with a filtered solution of Fat Red 7B (Sigma-Aldrich, http://www.sigmaaldrich.com), as previously described 11. For quantitative penetrance analysis, plants were grown and analyzed in triplicate from three plates for each experiment, and the experiment was repeated independently at least two times.
Starch staining
Roots from 1-month-old plants were cleared with chloral hydrate solution (8 g chloral hydrate, 2 ml glycerol, 1 ml H2O) and then stained with Lugol's solution (5 g I2, 10 g KI, 85 ml H2O).
RNA isolation and quantitative RT-PCR analysis
Total RNA was isolated using the NucleoSpin RNA Plant kit (Macherey-Nagel, http://www.mn-net.com). Quantitative RT-PCR was performed on a light cycler 480II (Roche), according to the manufacturer's instructions. Reaction volumes were scaled to 10 μl final volume and were comprised of 5 μl of SYBR Green PCR master mix (Roche, http://www.roche-applied-science.com), 2 μl of primer mix and 1 μl of template cDNA. Each sample was analyzed in triplicate, and PP2A, EXP and Tip41 were used as internal reference genes.
Yeast two-hybrid assay
The entire ORFs of the AtBMI1a, AtBMI1b and AtBMI1c cDNAs were amplified using gene-specific primers and subsequently cloned into the pGBKT7 and pGADT7 vectors (Clontech, http://www.clontech.com). Vectors containing LHP1, AtRING1a and AtRING1b have been previously described 7. Bait and prey constructs were cotransformed into the yeast strain pJ69-4a, and transformants were selected by growth on a synthetic defined (SD) medium lacking Leu and Trp (SD-LT). The bait-prey interaction was tested by growth of the transformants on SD medium lacking Leu, Trp and His plus 6 mM 3-amino-1,2,4-triazole (SD-LTH+3AT).
Pull-down assay
The AtBMI1a, AtBMI1b and AtBMIc cDNAs were cloned into the BamHI-XhoI sites of pGEX-4T-1 for production of GST-fused proteins. Production and purification of GST-AtBMI1a, GST-AtBMI1b and GST-AtBMI1c proteins were performed according to the manufacturer's recommendation (Amersham-Pharmacia Biotech, http://www.amersham.com). Pulldown experiments were performed as previously described 7.
References
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G . Genome regulation by polycomb and trithorax proteins. Cell 2007; 128:735–745.
Pien S, Grossniklaus U . Polycomb group and trithorax group proteins in Arabidopsis. Biochem Biophys Acta 2007; 1769:375–382.
Alvarez-Venegas R . Regulation by polycomb and trithorax group proteins in Arabidopsis. In: Chang C ed. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists, 2010.
Shen WH, Xu L . Chromatin remodeling in stem cell maintenance in Arabidopsis thaliana. Mol Plant 2009; 2:600–609.
Turck F, Roudier F, Farrona S, et al. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet 2007; 3:e86.
Zhang X, Germann S, Blus BJ, Khorasanizadeh S, Gaudin V, Jacobsen SE . The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat Struct Mol Biol 2007; 14:869–871.
Xu L, Shen WH . Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol 2008; 18:1966–1971.
Sanchez-Pulido L, Devos D, Sung ZR, Calonje M . RAWUL: a new ubiquitin-like domain in PRC1 ring finger proteins that unveils putative plant and worm PRC1 orthologs. BMC Genomics 2008; 9:308.
Qin F, Sakuma Y, Tran LS, et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell 2008; 20:1693–1707.
Masubelele NH, Dewitte W, Menges M, et al. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proc Natl Acad Sci USA 2005; 102:15694–15699.
Aichinger E, Villar CB, Farrona S, Reyes JC, Hennig L, Kohler C . CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet 2009; 5:e1000605.
Ogas J, Cheng JC, Sung ZR, Somerville C . Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science 1997; 277:91–94.
Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MA, de Vries SC . The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell 2003; 15:1563–1577.
Laux T, Mayer KF, Berger J, Jurgens G . The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996; 122:87–96.
Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T . Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell 2008; 14:867–876.
Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE . Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol 2003; 133:653–663.
Schmidt ED, Guzzo F, Toonen MA, de Vries SC . A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 1997; 124:2049–2062.
Boutilier K, Offringa R, Sharma VK, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002; 14:1737–1749.
Lotan T, Ohto M, Yee KM, et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 1998; 93:1195–1205.
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM . Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 1992; 4:1251–1261.
Luerssen H, Kirik V, Herrmann P, Misera S . FUSCA3 encodes a protein with a conserved VP1/AB13-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J 1998; 15:755–764.
Stone SL, Kwong LW, Yee KM, et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 2001; 98:11806–11811.
Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ . Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 2007; 12:245–252.
Blilou I, Xu J, Wildwater M, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005; 433:39–44.
To A, Valon C, Savino G, et al. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 2006; 18:1642–1651.
Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P . Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 1999; 20:503–508.
Zhang H, Rider SD Jr, Henderson JT, et al. The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J Biol Chem 2008; 283:22637–22648.
Chanvivattana Y, Bishopp A, Schubert D, et al. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 2004; 131:5263–5276.
Byrne ME, Barley R, Curtis M, et al. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000; 408:967–971.
Ori N, Eshed Y, Chuck G, Bowman JL, Hake S . Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 2000; 127:5523–5532.
Gaudin V, Libault M, Pouteau S, et al. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 2001; 128:4847–4858.
Exner V, Aichinger E, Shu H, et al. The chromodomain of LIKE HETEROCHROMATIN PROTEIN 1 is essential for H3K27me3 binding and function during Arabidopsis development. PLoS ONE 2009; 4:e5335.
Kotake T, Takada S, Nakahigashi K, Ohto M, Goto K . Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol 2003; 44:555–564.
Makarevich G, Leroy O, Akinci U, et al. Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 2006; 7:947–952.
Schubert D, Primavesi L, Bishopp A, et al. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J 2006; 25:4638–4649.
Clough SJ, Bent AF . Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Acknowledgements
We thank Lin Xu for generation of AtRING1a::AtRING1a-GUS transgenic plants and of Atring1a Atring1b as1 mutant, Francois Parcy (Laboratoire de physiologie cellulaire végétale, Grenoble) for providing the ABI3::GUS reporter line and Emily J McCallum for critical reading of this manuscript. AM is supported by a research-training fellowship from the Luxembourg Ministère de la Culture, de l'Enseignement Supérieur et de la Recherche. This work was funded by the French Centre National de la Recherche Scientifique (CNRS) and in part by the French Agence Nationale de la Recherche (ANR-08-BLAN-0200-CSD7).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Alignment of Arabidopsis, human and mouse BMI1 proteins. (PDF 904 kb)
Supplementary information, Figure S2
Severe phenotypes observed on 1-month-old Atbmi1a Atbmi1b plants before (A, C, E, G, I, K) and after Fat Red staining (B, D, F, H, J, L), respectively. Red staining indicates presence of embryonic traits. (PDF 4506 kb)
Supplementary information, Table S1
List of primers used in this study. (PDF 105 kb)
Rights and permissions
About this article
Cite this article
Chen, D., Molitor, A., Liu, C. et al. The Arabidopsis PRC1-like ring-finger proteins are necessary for repression of embryonic traits during vegetative growth. Cell Res 20, 1332–1344 (2010). https://doi.org/10.1038/cr.2010.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2010.151
Keywords
This article is cited by
-
Gametophytic epigenetic regulators, MEDEA and DEMETER, synergistically suppress ectopic shoot formation in Arabidopsis
Plant Cell Reports (2024)
-
Histone H2A monoubiquitination marks are targeted to specific sites by cohesin subunits in Arabidopsis
Nature Communications (2023)
-
Epigenetic modifications and miRNAs determine the transition of somatic cells into somatic embryos
Plant Cell Reports (2023)
-
Polycomb Repressive Complexes and Their Roles in Plant Developmental Programs, Particularly Floral Transition
Journal of Plant Biology (2023)
-
VAL1 acts as an assembly platform co-ordinating co-transcriptional repression and chromatin regulation at Arabidopsis FLC
Nature Communications (2022)