Abstract
Trogocytosis is a rapid transfer between cells of membranes and associated proteins. Trogocytic exchanges have been investigated between different cell types, mainly in two-cell systems, involving one donor and one acceptor cell type. Here, we studied trogocytosis in a more complex system, involving not only several immune cell subsets but also multiple tumor cells. We show that CD4+ T cells, CD8+ T cells and monocytes can acquire membrane patches and the intact proteins they contain from different tumor cells by multiple simultaneous trogocytoses. The trogocytic capabilities of CD4+ and CD8+ T cells were found to be similar, but inferior to that of autologous monocytes. Activated peripheral-blood mononuclear cells (PBMCs) may also exchange membranes between themselves in an all-autologous system. For this reason, monocytes are capable of acquiring membranes from multiple tumor cell sources, and transfer them again to autologous T cells, along with some of their own membranes (serial trogocytosis). Our data illustrate the extent of membrane exchanges between autologous activated immune effector cells and their environment, and how the cellular content of the local environment, including “bystander” cells, may impact the functions of immune effector cells.
Similar content being viewed by others
Introduction
The immune system is constituted of cell types that interact with each other and each have specific functions. For instance, dendritic cells, and to a lesser extent monocytes, can act as antigen-presenting cells (APCs), and stimulate CD4+ T cells to become T-helper cells and CD8+ T cells to become cytotoxic effector cells. Such mechanism depends, for a significant part, on interactions between ligands and receptors expressed at the cell surface of both cell types, such as MHC-II:TCR, MHC-I:TCR or CD28:CD80 interactions. Thus, the functions of immune cells depend on the molecules expressed at their surface, which enable them to interact with other cells 1, 2.
The phenotype of immune cells, including the expression of molecules involved in immune functions is considered to be fixed and characteristic of each of the cell subsets. From a basic phenotype, one can therefore deduce function. However, under certain circumstances, membranes and/or membrane-associated proteins such as MHC proteins can be transferred between cells by a mechanism called trogocytosis, enabling the “acceptor” cell to display proteins borrowed from the “donor” cell (reviewed in 3).
Trogocytosis is a relatively new name for a unidirectional and active transfer of membranes and associated molecules from one cell to another 4. The characteristics of trogocytosis are cell-to-cell contact requirement, fast kinetics and limited lifetime of the transferred molecules at the surface of the acceptor cells. In humans, trogocytic transfers have been described between APCs and T cells 5, 6, 7, tumor cells and NK cells 8, 9, 10, NK cells and B cells 9, γδ T cells and tumor cells 11, and dendritic cells and tumor cells 12. Functionally, trogocytic transfers have been shown to temporarily endow the acceptor cells with some functions of the donor cells. For instance, (i) CD8+ T cells that acquired their cognate MHC class I + peptide ligands from their targets became susceptible to “fratricide” antigen-specific cytolysis 13, 14; (ii) T cells which had acquired HLA-DR and CD80 from APCs could stimulate resting T cells in an antigen-specific manner, and thus behaved as APCs themselves 15, 16, 17 and (iii) CD4+ T cells and NK cells which had acquired HLA-G behaved as suppressor cells for other T and NK cells 7, 10.
HLA-G (reviewed in 18) is characterized by a very low amount of polymorphism and an expression which is restricted to fetal trophoblast cells, adult thymic medulla, cornea, erythroid precursors, pancreas and mesenchymal stem cells. However, HLA-G can be neo-expressed in transplantation 19, inflammatory diseases 20, cancers 21 and viral infections 22. HLA-G is a potent tolerogenic molecule that inhibits the cytolytic functions of NK 23 and T cells 24, CD4+ T cell alloproliferation 25, the ongoing proliferation of T cells 7, 26 and NK cells 10, and dendritic cell maturation 27 and it induces regulatory T cells 28.
We previously demonstrated that HLA-G transfer from HLA-G1-transfected APCs to CD4+ T cells, and from HLA-G-transfected tumor cells to NK cells in vitro changed the function of the effector cells to the point of inversion. Indeed, after trogocytosis of HLA-G1-containing membranes, these effector cells behaved as temporary regulatory cells capable of blocking allogeneic responses and allo-cytotoxicity using the acquired HLA-G1 7, 10. From these data, we hypothesized that, by transferring HLA-G1-containing membranes onto effector cells, a few HLA-G1-expressing cells might propagate HLA-G1-dependent immune suppression. Through trogocytosis, a few HLA-G1-expressing cells would (i) quickly increase the number of regulatory HLA-G1-positive cells in their vicinity without unlocking HLA-G1 expression and (ii) spread HLA-G1 presence to a larger area. This would block the function of any effector cell in that area, and dampen/stop the local reaction, preventing damages to tissues in the vicinity of HLA-G1-expressing cells.
With the exception of in vivo experiments 8, 29, the mechanism of trogocytic exchanges has been investigated mainly in two-cell systems involving one donor and one acceptor cell type. Here, we investigated the extent of trogocytic exchanges in a less simplified system involving several immune effector cell types (CD4+ T cells, CD8+ T cells and monocytes) and two different cell lines, which represent environmental non-immune cells. Our data show that membranes, including some that contain HLA-G, are extensively acquired from one or several cell types by activated CD4+ T cells, activated CD8+ T cells and, with an even higher efficiency, by activated monocytes. This acquisition may be direct, following a cell-to-cell contact between acceptor immune cells and donor tumor cells, or may involve a third party cell as an intermediate. Indeed, we demonstrated that monocytes that had acquired membranes from tumor cells could transfer them again, along with patches of their own plasma membranes. These data strengthen the notion that membrane-bound antigen exchanges are extensive in the context of immune activation and seem to be a normal feature of the interaction between activated immune cells and their surroundings.
Results
Activated CD4+, CD8+ T cells and CD14+ monocytes from peripheral blood acquire tumor cell membranes by trogocytosis
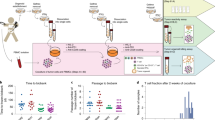
We first evaluated the extent of trogocytosis between activated immune cells and the two HLA-G1-transfected tumor cell lines used in this study, i.e., melanoma M8-HLA-G1 cells and lymphoblastoid LCL-HLA-G1 cells. Figure 1A demonstrates that PHA-L-activated CD4+ T cells, CD8+ T cells and CD14+ monocytes were capable of acquiring HLA-G1-containing membranes from either PKH67-labelled M8-HLA-G1 cells (M8-HLA-G167 cells) or PKH26-labelled LCL-HLA-G1 cells (LCL-HLA-G126 cells). Indeed, after a 30-min co-incubation, effector immune cells became positive for donor membranes (PKH) and HLA-G. In these experiments, HLA-G1 was used to demonstrate that membrane-bound proteins transfer along with PKH-labelled lipids. However, it should be stressed that HLA-G1 is but one of the proteins transferred, as previously reported 7. Activation of the acceptor cells greatly enhanced their trogocytic capabilities in our system, even though it is otherwise possible to observe membrane transfer between APCs and allogeneic T cells or monocytes 5, 6, 30. In our system, after co-incubating non-activated PBMCs with LCL-HLA-G1 cells, 16% of CD4+ T cells and 11% of CD8+ T cells were HLA-G-positive. However, after co-incubation of PHA-L-activated PBMCs, the percentages increased to 73% of CD4+ T cells and 60% of CD8+ T cells (data not shown).
Monocytes and T cells acquire HLA-G-containing membranes from different tumor cells by trogocytosis. PKH and/or HLA-G staining (detected with MEM-G/09 antibody) was evaluated by flow cytometry on CD4+ T cells, CD8+ T cells and CD14+ monocytes. (A) Analysis prior to and after co-incubation with PKH67-labelled M8-HLA-G1 cells (M8-HLA-G167, top) or with PKH26-labelled LCL-HLA-G1 cells (LCL-HLA-G126, bottom). Percentages of positive cells for each staining are indicated. Data are representative of 15 independent experiments. (B) Statistical analysis of trogocytosis efficiency in CD4+ T cells (n = 11) compared with trogocytosis efficiency in CD8+ T cells (n = 12) for each type of donor cells. (C) Statistical analysis of trogocytosis efficiency in T cells (n = 22) compared with trogocytosis efficiency in monocytes (n = 9) for each type of donor cells.
In order to ascertain a trogocytic mechanism, we first demonstrated that cell-to-cell contact was necessary by separating donor and acceptor cells by a transwell membrane (Supplementary information, Figure S1A). In the presence of the transwell membrane, acceptor cells did not acquire membranes. Next, we co-incubated activated PBMCs with M8 cells expressing HLA-G1 fused to EGFP in its intracellular part (M8-HLA-G1-GFP cells) and showed that after co-incubation, CD4+ T cells, CD8+ T cells and CD14+ monocytes displayed the full-length EGFP-HLA-G1 protein, and not a shed or an endogenously-produced one (Supplementary information, Figure S1B).
Masking HLA-G or its receptor ILT2 with blocking antibodies had no effect on trogocytosis, ruling out the involvement of HLA-G:ILT2 interaction in these transfers (data not shown). Moreover, even though MHC:TCR interactions are involved in trogocytic transfers in other systems 5, 6, 13, this was not the case in ours where activated effectors were used, as already reported 7, 10. Indeed, CD4+ and CD8+T cells acquired membranes from both HLA-Ipos/HLA-IIneg M8-HLA-G1 cells and HLA-Ineg/HLA-IIpos LCL-HLA-G1 cells to a similar extent (Figure 1B). Lastly, activated monocytes were two (LCL-HLA-G1 donor cells) to five (M8-HLA-G1 donor cells) times more efficient at capturing membranes than autologous activated T cells (Figure 1C).
Figure 2 illustrates, by confocal microscopy, the direct trogocytic transfers between PKH26-labelled M8-HLA-G1-EGFP cells (M8-HLA-G1-EGFP26 cells) and CD4+, CD8+ and CD14+ cells. One can see that, prior to co-incubation, CD14+ monocytes, CD4+ T cells and CD8+ T cells were negative for GFP and PKH fluorescence, and did not express HLA-G. After a 30-min co-incubation with M8-HLA-G1-EGFP26 cells, all three immune subsets had acquired patches of M8 membranes containing intact HLA-G molecules, as shown by the co-localization of PKH26 fluorescence of M8 membranes, with the EGFP fluorescence of HLA-G intracellular domain, and with the signal of HLA-G extracellular domain.
Intact HLA-G-containing membrane patches are acquired by monocytes and T cells from tumor cells by trogocytosis. Confocal microscopic analysis of membranes acquired by activated PBMC subsets before and after co-incubation with M8-HLA-G1-GFP26 cells. Green: EGFP fluorescence. Red: PKH26 fluorescence. Light blue: HLA-G. Dark blue: CD4, CD8 or CD14 subset marker.
Taken together, these data show that activated immune cell subsets acquired membrane patches and their associated intact molecules from tumor cells by trogocytosis.
Multiple trogocytosis: immune cells acquire membranes from multiple sources simultaneously
In order to determine to which extent activated immune cells captured membranes from multiple sources in their environment, we co-incubated CD4+ T cells, CD8+ T cells and CD14+ monocytes with both M8-HLA-G167 and LCL-HLA-G126 cells. Figure 3A shows that activated CD4+ T cells, CD8+ T cells and CD14+ monocytes were all capable of capturing membranes from both sources. Figure 3A also shows that cells which acquired membranes from multiple sources are not a minor population, but represent half of the trogocytic cell population, considering the trogocytic population as the cells that have acquired membranes from at least one donor cell type. In these experiments CD4+ T and CD8+ T cells again showed an identical efficiency in acquiring membrane from HLA-Ipos/HLA-IIneg M8-HLA-G167 cells and from HLA-Ineg/HLA-IIpos LCL-HLA-G126 cells, which confirms that trogocytosis by already activated T cells does not depend on MHC:TCR interactions.
Monocytes and T cells acquire membrane patches from multiple tumor sources. PKH and/or HLA-G staining was evaluated on PHA-activated PBMC subsets after co-incubation with both M8-HLA-G167 cells and LCL-HLA-G126 cells simultaneously. (A, top) Flow cytometry analysis of membrane donor cells prior to co-incubation. (A, bottom) Flow cytometry analysis of PHA-activated PBMC subsets prior to and after co-incubation with membrane donor cells. Percentages of positive cells for each staining are indicated. Data are representative of six independent experiments. (B) Confocal microscopic analysis of PBMC subsets after co-incubation with membrane donor cells. Green: PKH67 fluorescence. Red: PKH26 fluorescence. Light blue: HLA-G. Dark blue: CD4, CD8 or CD14 subset marker.
Figure 3B shows, by confocal microscopy, representative examples of CD4+ T cells, CD8+ T cells and CD14+ monocytes that acquired membranes from both M8-HLA-G167 and LCL-HLA-G126 cells. In this figure, several membrane patches of M8-HLA-G167 or LCL-HLA-G126 origin can be seen at the surface of the acceptor cells, some of which contain HLA-G1.
Taken together, these experiments demonstrate that activated immune cells can take up multiple membrane patches from multiple sources, possibly simultaneously.
Serial trogocytosis: monocytes can acquire membranes from tumor cells and transfer them again to autologous immune cells
We have shown that T cells and monocytes could acquire membrane fragments from allogeneic tumor cells by trogocytosis. We next investigated whether membranes directly acquired from the donor cells by monocytes could be transferred again to autologous cells.
Trogocytosis from APCs to effector cells is well described in allogeneic and/or antigen-dependent contexts 5, 6, 7, but much less in autologous contexts 31. Thus, we first investigated whether membrane transfers between monocytes and other activated immune effectors occurred in an autologous context and in the absence of immunizing antigen. For this purpose, we co-incubated PKH67-labelled monocytes (CD1467 cells) with autologous purified CD4+ T cells, CD8+ T cells or CD14+ monocytes. In this system, membrane donor monocytes (CD1467 cells) were clearly distinguishable from T cells using lineage markers and from autologous monocytes because of their high level of PKH fluorescence. Figure 4 shows that after co-incubation with CD1467 monocytes, activated autologous CD4+ T cells (44%), CD8+ T cells (48%) and CD14+ monocytes (79%) had acquired PKH67 fluorescence. This demonstrates that membrane transfers between monocytes and other activated immune cells occur in an autologous context and in the absence of immunizing antigen.
Monocytes can act as membrane donor cells for autologous activated PBMCs. Flow cytometry analysis of membrane transfers from PKH67-labelled activated monocytes (CD1467) to autologous PHA-activated PBMCs. Top: PKH67 fluorescence on CD1467 prior to co-incubation. Bottom: PKH67 fluorescence on CD4+ T cells, CD8+ T cells and monocytes prior to and after a 30-min co-incubation with CD1467 cells. Percentages of PKH67-positive cells are indicated. Data are representative of five independent experiments.
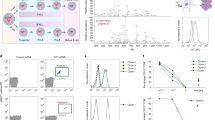
Next, we investigated whether monocytes could retransfer membranes previously acquired from other cells. For this, we first co-incubated LCL-HLA-G126 cells with activated monocytes that had been labelled with PKH67 and coated with anti-CD14 magnetic beads. This “primary trogocytosis” led to the generation of membrane “secondary donors” (CD1467,(26acq+) cells i.e. CD1467 monocytes which had acquired PKH26-labelled membranes from LCL-HLA-G126 cells). Being coated with anti-CD14 magnetic beads, secondary donor cells were then positively purified from primary donors using magnetic cell sorting. Primary and secondary membrane donors are represented in Figure 5A. In the second part of the experiment, CD1467,(26acq+) cells were co-incubated with autologous activated PBMCs, and the membrane transfer to these secondary acceptors (CD4+ T cells, CD8+ T cells and CD14+ monocytes) of endogenous monocytic membranes (PKH67) or membranes previously acquired from LCL-HLA-G126 (PKH26) was evaluated by flow cytometry. Figure 5A shows that following this secondary trogocytosis, secondary acceptor cells from all three subsets had acquired both PKH67-labelled membranes from CD1467 monocytes themselves (secondary donors), and PKH26-labelled membranes of LCL-HLA-G126 origin, which had been previously acquired by CD1467 monocytes during the primary trogocytosis. Interestingly, monocytes and lymphocytes differed as secondary acceptor cells. Indeed, all monocytes that had acquired membranes from secondary donors had also acquired membranes originally captured from primary donor cells. By contrast, only a minority of trogocytic lymphocytes that acquired membranes from CD1467 monocytes also acquired PKH26-labelled membranes originally from primary donor cells (45% for CD4+ T cells and 21% for CD8+ T cells in the experiment presented).
Through serial trogocytosis, monocytes can redistribute HLA-G-containing membranes acquired from tumor cells to autologous immune cells. The presence of HLA-G and/or PKH-labelled membranes was investigated on activated PBMC subsets after co-incubation with PKH67-labelled monocytes that had previously acquired membranes from PKH26-labelled HLA-G+tumor cells. (A, top) Flow cytometry analysis of LCL-HLA-G126 cells and PKH67-labelled monocytes prior to (CD1467) and after (CD1467,(26acq+)) the co-incubation (primary trogocytosis). (A, bottom) Flow cytometry analysis of PBMC subsets prior to and after co-incubation with CD1467,(26acq+) monocytes (secondary trogocytosis). Percentages of positive cells for each staining are indicated. Data are representative of eight independent experiments. (B) Confocal microscopic analysis of PBMC subsets after secondary trogocytosis. Green: PKH67 fluorescence. Red: PKH26 fluorescence. Light blue: HLA-G. Dark blue: CD4, CD8 or CD14 subset marker.
Figure 5B is a confocal microscopy image of representative CD4+, CD8+ and CD14+ cells which had acquired both PKH26-labelled and PKH67-labelled membranes from CD1467,(26 acq+) monocytes. Patches labelled only with PKH67 can be observed, corresponding to the acquisition of secondary donor membranes only. Patches labelled with both PKH67 and PKH26 can also be observed, indicating that membranes of LCL-HLA-G1 origin and membranes of monocytic origin had been taken up simultaneously. In these experiments, HLA-G, originally present only at the surface of primary donor cells, was also transferred to secondary acceptor cells and co-localized with PKH26 fluorescence, which confirms its LCL-HLA-G126 origin.
In order to gain insight into the mechanisms and parameters of serial trogocytosis, we compared trogocytosis efficiencies between direct and secondary trogocytosis, and between trogocytosis performed by different cell types. We first compared the amounts of primary donor cell membranes transferred during the course of these two co-incubations. Figure 6A shows that the amount of primary donor cell membranes to be acquired by the three cell subsets is higher in direct than in secondary trogocytosis. This was expected since during secondary trogocytosis, the only primary donor cell membranes available are those that are already acquired by the secondary donor cells. We next investigated whether allogeneic membranes have an impact on the secondary trogocytosis. Figure 6B shows that the proportions of monocytic membranes acquired by activated CD4+ T cells, CD8+ T cells and CD14+ monocytes from autologous monocytes, or from autologous monocytes which had previously acquired membranes from M8-HLA-G1 cells (monocytesM8-HLA-G1_mb_acq+), or from LCL-HLA-G1 cells (monocytesLCL-HLA-G1_mb_acq+) were not significantly different. Lastly, we investigated which membranes were preferentially acquired during secondary trogocytosis by the effector cells: endogenous membranes from the secondary donor monocytes or membranes that monocytes had previously acquired from LCL-HLA-G1 cells. Interestingly, we found that all three secondary acceptor cell subsets preferentially acquired membrane patches that contained LCL-HLA-G1 membranes (Figure 6C).
Statistical analysis of trogocytosis efficiencies. (A) Efficiency of direct trogocytosis (white bars, n = 11) and secondary trogocytosis (gray bars, n = 10) for each type of acceptor cells (CD4+ T cells, CD8+ T cells and CD14+ monocytes) Results are indexed on the fluorescence of the tumor cells. (B) Impact of the presence of allogeneic membranes acquired during primary trogocytosis on the efficiency of secondary trogocytosis by CD4+, CD8+ or CD14+ cells. Membrane donor cells were control monocytes (white bars, n = 5), monocytes that had previously acquired membranes from M8-HLA-G167 cells (gray bars, n = 3), or monocytes that had previously acquired membranes from LCL-HLA-G126 cells (black bars, n = 8). Results are indexed on the fluorescence of the monocytes. (C) Secondary trogocytosis efficiency for the transfers of endogenous monocyte membranes (white bars, n = 8) and allogeneic membranes acquired from LCL-HLA-G126 cells (gray bars, n = 8).
Multiple and serial trogocytosis: monocytes can acquire membranes from several tumor cells and transfer them again to autologous immune cells
We finally investigated whether multiple and serial trogocytosis was possible. For this purpose, we incubated unlabelled monocytes attached to magnetic beads, with M8-HLA-G167 and LCL-HLA-G126 cells, generating monocytes with PKH67-labelled patches of M8-HLA-G167 origin and PKH26-labelled patches of LCL-HLA-G126 origin (CD14(67acq+,26acq+)). Primary and secondary membrane donors are represented in Figure 7. After primary trogocytosis, we purified the CD14(67acq+,26acq+) monocytes by magnetic cell sorting and co-incubated them with autologous activated PBMCs (secondary trogocytosis). Figure 7 shows that following secondary trogocytosis, some cells from all three secondary acceptor cell subsets had acquired both PKH67-labelled and PKH26-labelled membranes of M8-HLA-G167 and LCL-HLA-G126 origin, respectively. This demonstrates that multiple and serial trogocytosis is possible, i.e. monocytes can acquire membranes from several cellular sources and transfer them again to other activated immune cells.
Multiple and serial trogocytosis: monocytes can redistribute membranes acquired from several tumor cells to autologous immune cells. PKH staining was evaluated by flow cytometry on activated PBMC subsets after a co-incubation with monocytes that had previously acquired membranes from several tumor cells simultaneously. Top: flow cytometry analysis of primary membrane donor cells (M8-HLA-G167 cells and LCL-HLA-G126 cells) and of monocytes coated with anti-CD14 magnetic beads prior to (CD14) and after (CD1467acq+,26acq+) the co-incubation with primary donor cells (primary trogocytosis). Bottom: flow cytometry analysis of PBMC subsets prior to and after co-incubation (secondary trogocytosis) with CD1467acq+,26acq+ monocytes. Percentages of positive cells for each staining are indicated. Data are representative of three independent experiments.
Discussion
In this work, we investigated the extent of the trogocytic transfers of membranes and associated molecules between immune and non-immune cells. We first demonstrated that several simultaneous and independent trogocytic processes can occur between multiple non-immune cells acting as donor cells, and one acceptor immune cell. We further showed that serial trogocytosis, and even multiple serial trogocytosis, is possible since monocytes could redistribute to autologous activated PBMCs, membrane fragments that had been acquired from one or multiple tumor cells through a previous allogeneic trogocytosis. Taking all these results together, we suggest that trogocytosis is not such a rare phenomenon, but a regular feature of immune processes.
Some studies reported that an MHC:TCR interaction was needed for trogocytosis between T cells and APCs 5, 6, 13, while others have shown that trogocytosis may occur through interactions other than MHC:TCR 9, 16, 32. In our system, antigen-specific interaction was apparently not essential for a successful transfer. Indeed, (i) CD4+ T cells and CD8+ T cells had a similar efficiency in taking up membranes from tumor cells, independently of the HLA-class I or II expression by donor cells and (ii) successful trogocytosis was achieved between immune cells in an all-autologous system. This rules out an impact of primary donor allo-antigens on the membrane transfer described here. Thus, it seems that MHC:TCR interaction itself is not absolutely required for the trogocytic membrane antigen capture by activated cells, even if it may be required for the trogocytic membrane capture by resting T cells. In our opinion, MHC:TCR interaction does not play a key role in trogocytosis by activated cells, whereas activation does 7, 10, 11, 16, 30. However, one can argue that since in most systems that have been used to study trogocytosis, activation is achieved through MHC:TCR interaction, cognate recognition of antigen through TCR is, in a way, mandatory for trogocytosis, even for trogocytosis by activated effector cells.
As demonstrated by the strict requirement for intercellular contact, cell-to-cell interactions other than MHC:TCR interactions are required for trogocytosis. These cell-to-cell interactions are not clearly defined, may be multiple and redundant, and may differ depending on the donor:acceptor cell pair. In this work and in others previously published 7, 10, 30, antibodies blocking the HLA-G:ILT2 interaction had no effect on the efficiency of trogocytosis, ruling out the involvement of HLA-G in the trogocytic processes we observed.
Confocal images of acceptor cells after trogocytosis showed that trogocytic processes that occurred during the 30-min time frame of our studies were most likely independent from each other, as shown by individualized membrane fragments at the surface of most of the acceptor cells. Yet, trogocytosis efficiency calculations revealed that membranes that had been previously acquired by monocytes from tumor cells were preferentially transferred again to other cells. This might mean that membranes collected by monocytes were not randomly distributed at the monocytes' surface but located in a clustered manner possibly at sites that are trogocytosis-prone.
When activated immune cells were co-incubated at the same time with different non-immune cells several trogocytic processes were observed simultaneously resulting in the transfer of membranes from several sources to one acceptor cell. To our knowledge, this is the first report of multiple trogocytosis. This finding is noteworthy because, while experiments in two-cell systems have proved trogocytosis, described its parameters, and elucidated some of its key mechanisms, multiple trogocytosis highlights the qualitative extent of this mechanism. It suggests that membrane exchanges between activated immune effector cells and the cells surrounding them are extensive and somewhat non-discriminative. Given what is known of the transfer of functional proteins through trogocytosis, our results emphasize the potential role of bystander cells in immune responses.
Throughout our studies, monocytes were more efficient than T cells at taking up membranes from different sources, including from other autologous immune cells, and at redistributing them. This might be linked to the antigen collecting/processing/presenting functions of these cells. In that case, it may be considered that monocytes have the capability to take up membrane-bound antigens from living cells in order to (i) process them for presentation in the context of class I and class II, (ii) temporally display them and possibly use them as unprocessed molecules which may alter their function, as in the case of HLA-G, and (iii) redistribute these collected membrane antigens from multiple sources to other immune cells.
Since trogocytosis seems to be a common phenomenon allowing intercellular transfer of intact and functional molecules, trogocytic processes could be considered as an important mechanism to spread the antigen display and to enable cells to act through molecules they do not express. This is especially important for molecules with a limited tissue distribution such as HLA-G. For these molecules, serial trogocytosis can be considered as a mechanism to amplify their expression and effects. This means that a few HLA-G-positive tumor cells may generate a larger number of HLA-G-positive cells in a short time without the need for cell maturation or unlocking HLA-G expression. This is relevant because HLA-G plays a key role in immunotolerance to the fetus and its expression has been demonstrated in a variety of tumors (reviewed in 18), contributing to the escape from the immune attack 33, 34. Thus, HLA-G trogocytosis, whether it is direct, multiple and/or serial, may further amplify HLA-G tolerogenic effect. In the present study, we could not perform functional assays after secondary trogocytosis because of technical considerations. However, this hypothesis is based on in vitro data showing that when transferred by direct trogocytosis, HLA-G maintains its functionality in the acceptor cells and allows them to act, at least temporarily, as if they endogenously expressed it 7, 10, 30.
Taken together, our results indicate that trogocytosis could play an important role in the immune response through the spreading of membrane-associated proteins such as HLA-G, or through the influence of the surrounding non-immune cells on immune cells.
Materials and Methods
Cells and cell lines
Blood was obtained from healthy volunteer donors through the French Blood Bank under a protocol approved by the Institutional Review Board of the Saint Louis Hospital, Paris. PBMCs were isolated on Histopaque 1077 density gradient (Sigma, St Louis, MO, USA).
Monocytes were isolated either by adherence, or by magnetic cell sorting. For magnetic cell sorting, Fc receptors of PBMC were first blocked using 20 μg/ml human IgG (Sigma) for 30 min. A total of 20 μg/ml of anti-CD14 were then added and CD14-positive monocytes were positively separated using goat anti-mouse-coated magnetic beads according to the manufacturer's specifications (Ademtech, France).
Adherent melanoma cell line M8 transfected with pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) alone (M8-pcDNA), containing HLA-G1 cDNA (M8-HLA-G1) or containing cDNA of HLA-G1 fused to EGFP (M8-HLA-G1-GFP); lymphoblastoid LCL-721.221 cells (LCL) transfected with pRc/RSV vector (Invitrogen) alone (LCL-RSV) or containing HLA-G1 cDNA (LCL-HLA-G1) have been previously described 7, 10.
All cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated FCS.
Cell activation
Purified monocytes were activated with 100 ng/ml lipopolysaccharide (Sigma) for 48 h. PBMCs or purified T cells were activated for 48 h with 4 μg/ml leucoagglutinin (PHA-L; Sigma) and were cultured in IL-2-supplemented medium (100 U/ml; Sigma).
Membrane labelling
Cell membrane labelling was performed using either PKH67 or PKH26 Fluorescent Cell Linker Mini kit (Sigma) according to the manufacturer's specifications.
Transwell experiments
Transwell experiments were performed using Transwell culture system (Greiner Bio-One, Kremsmünster, Austria): immune cells were cultured in the upper chamber of a 12-well plate and separated from M8-HLA-G167 and LCL-HLA-G126 cells by a 0.4-μm pore semipermeable membrane.
Trogocytosis assays
Direct/primary trogocytosis: activated monocytes, T cells or PBMCs were co-incubated with PKH67-labelled M8-HLA-G1 cells (M8-HLA-G167 cells) or with PKH26-labelled LCL-HLA-G1 cells (LCL-HLA-G126 cells) at a 1:1 ratio for 15-30 min at 37 °C and 5% CO2. Even though M8 cells are adherent, trogocytosis assays were performed in cell suspensions. After co-incubation (trogocytosis), cells were placed on ice and all further steps were performed on ice.
Multiple trogocytosis: activated PBMCs were co-incubated with both M8-HLA-G167 cells and LCL-HLA-G126 cells at the same time and in the same conditions as above.
Serial trogocytosis: a primary trogocytosis was first performed by co-incubating PKH26-labelled LCL-HLA-G1 cells (LCL-HLA-G126) with activated PKH67-labelled monocytes (CD1467) attached to magnetic beads. During this co-incubation, monocytes acquired PKH26-labelled membranes from LCL-HLA-G1 cells (CD1467,(26acq+) cells; primary trogocytosis). Next, these monocytes were purified by magnetic cell sorting. After verifying by flow cytometry that all LCL-HLA-G126 cells had been removed, these CD1467,(26acq+) monocytes were used as “secondary donor cells” in a co-incubation with autologous PHA-activated PBMCs (secondary trogocytosis), in the same conditions as for primary trogocytosis. Secondary trogocytosis was only performed when contamination by LCL-HLA-G126 cells was less than 1%. The exact same experiments were performed using PKH26-labelled M8-HLA-G126 in suspension as primary donor cells.
Multiple and serial trogocytosis: for this experiment, PHA-L-activated unlabelled monocytes attached to magnetic beads (CD14) were co-incubated with PKH67-prelabelled M8-HLA-G1 cells (M8-HLA-G167) and with PKH26-prelabelled LCL-HLA-G1 cells (LCL-HLA-G126). This generated “secondary donor monocytes” with PKH67-labelled patches of M8-HLA-G167 origin and PKH26-labelled patches of LCL-HLA-G126 origin (CD14(67acq+,26acq+)). CD14(67acq+,26acq+) monocytes were then positively purified by magnetic cell sorting, and the absence of primary donor cells from the sorted population was verified by flow cytometry. CD14(67acq+,26acq+) monocytes were then used as membrane donor cells for autologous activated PBMC in a secondary trogocytosis assay.
Trogocytosis results were evaluated by flow cytometry and confocal microscopy. For the latter, monocytes attached to magnetic beads were removed from the co-culture after trogocytosis, for a better visualization of the final membrane acceptor cells.
Antibodies and flow cytometry
All antibodies were from Exbio (Prague, Czech Republic): FITC-conjugated, PE-conjugated or biotin-conjugated non-blocking anti-HLA-G Mem-G/09; purified or biotin-conjugated blocking anti-HLA-G 87G; purified or PE-DY647-conjugated anti-CD4, anti-CD8, anti-CD14. Flow cytometry analyses were performed on an EPICS XL Cytometer (Beckman Coulter, Fullerton, CA, USA). Prior to staining, Fc receptors were blocked with 20 μg/ml human IgG. Appropriate isotypic controls were systematically used to evaluate non-specific binding. Membrane transfer was directly analyzed through PKH fluorescence.
Confocal microscopy
Membrane transfer was visualized using PKH fluorescence. Acceptor cells of each subset were identified with mouse anti-CD4 (IgG1 antibody), anti-CD8 (IgG2a antibody) or anti-CD14 monoclonal antibodies, respectively, and HLA-G was visualized using biotinylated anti-HLA-G antibodies. To avoid cross-reactivity between HLA-G staining and lineage marker staining, we used 87 G, an IgG2a antibody, to detect HLA-G in CD4+ T cells and monocytes, whereas MEM-G/09, an IgG1 antibody, was used to detect HLA-G in CD8+ T cells. In a second step, streptavidin-Alexa405 and a goat anti-mouse (anti-IgG1 or anti-IgG2a) antibody conjugated to Alexa647 (Invitrogen) were used as secondary antibodies.
After the staining, cells were left adhered on poly-L-lysine-coated slides for 3 min at 37 °C and fixed for 15 min with 3% paraformaldehyde (Sigma). Samples were finally mounted in a VECTASHIELD mounting medium (Clinisciences, Paris, France) and analyzed using a Carl Zeiss LSM 510 confocal microscope (Zeiss, Germany).
Statistical analysis
To compare different types of trogocytosis, or trogocytosis performed between different cell types, we calculated the trogocytosis efficiency in each experiment using mean fluorescence intensity (MFI) values for the PKH fluorescence.
Trogocytosis efficiency was calculated as: ((MFI of acceptor cells after trogocytosis−MFI of acceptor cells before trogocytosis)/MFI of donor cells)×100.
Thus, the amount of transferred membranes to the acceptors was normalized to the amount of membranes of donor cells originally available.
We performed the statistical analysis of this parameter with SPSS Program for Windows. Comparisons of trogocytosis efficiencies were performed using the non-parametric Mann-Whitney U test. P values less than 0.05 were considered to be significant.
References
Medzhitov R . Recognition of microorganisms and activation of the immune response. Nature 2007; 449:819–826.
Buckwalter MR, Albert ML . Orchestration of the immune response by dendritic cells. Curr Biol 2009; 19:R355–361.
Davis DM . Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol 2007; 7:238–243.
Joly E, Hudrisier D . What is trogocytosis and what is its purpose? Nat Immunol 2003; 4:815.
Arnold PY, Davidian DK, Mannie MD . Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur J Immunol 1997; 27:3198–3205.
Huang JF, Yang Y, Sepulveda H, et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 1999; 286:952–954.
LeMaoult J, Caumartin J, Daouya M, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007; 109:2040–2048.
Sjostrom A, Eriksson M, Cerboni C, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med 2001; 194:1519–1530.
Carlin LM, Eleme K, McCann FE, Davis DM . Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med 2001; 194:1507–1517.
Caumartin J, Favier B, Daouya M, et al. Trogocytosis-based generation of suppressive NK cells. EMBO J 2007; 26:1423–1433.
Espinosa E, Tabiasco J, Hudrisier D, Fournie JJ . Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J Immunol 2002; 168:6336–6343.
Zhang QJ, Li XL, Wang D, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS One 2008; 3:e3097.
Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E . Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol 2001; 166:3645–3649.
Hudrisier D, Riond J, Garidou L, Duthoit C, Joly E . T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol 2005; 35:2284–2294.
Game DS, Rogers NJ, Lechler RI . Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant 2005; 5:1614–1625.
Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H . Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol 2002; 169:6162–6169.
Xiang J, Huang H, Liu Y . A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol 2005; 174:7497–7505.
Carosella ED, Favier B, Rouas-Freiss N, Moreau P, LeMaoult J . Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 2008; 111:4862–4870.
Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED . Implication of HLA-G molecule in heart-graft acceptance. Lancet 2000; 355:2138.
Wiendl H, Behrens L, Maier S, Johnson MA, Weiss EH, Hohlfeld R . Muscle fibers in inflammatory myopathies and cultured myoblasts express the nonclassical major histocompatibility antigen HLA-G. Ann Neurol 2000; 48:679–684.
Paul P, Rouas-Freiss N, Khalil-Daher I, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA 1998; 95:4510–4515.
Lozano JM, Gonzalez R, Kindelan JM, et al. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. Aids 2002; 16:347–351.
Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED . The alpha 1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci USA 1997; 94:5249–5254.
Le Gal F-A, Riteau B, Sedlik C, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol 1999; 11:1351–1356.
Riteau B, Menier C, Khalil-Daher I, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol 1999; 43:203–211.
Bahri R, Hirsch F, Josse A, et al. Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol 2006; 176:1331–1339.
Ristich V, Liang S, Zhang W, Wu J, Horuzsko A . Tolerization of dendritic cells by HLA-G. Eur J Immunol 2005; 35:1133–1142.
LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED . HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA 2004; 101:7064–7069.
Zimmer J, Ioannidis V, Held W . H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function. J Exp Med 2001; 194:1531–1539.
HoWangYin KY, Alegre E, Daouya M, Favier B, Carosella ED, Lemaoult J . Different functional outcomes of intercellular membrane transfers to monocytes and T cells. Cell Mol Life Sci 2010; 67:1133–1145.
Poupot M, Fournie J-J, Poupot R . Trogocytosis and killing of IL-4-polarized monocytes by autologous NK cells. J Leukoc Biol 2008; 84:1298–1305.
Hwang I, Huang JF, Kishimoto H, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med 2000; 191:1137–1148.
Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H . HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood 2005; 105:1694–1698.
Wagner SN, Rebmann V, Willers CP, Grosse-Wilde H, Goos M . Expression analysis of classic and non-classic HLA molecules before interferon alfa-2b treatment of melanoma. Lancet 2000; 356:220–221.
Acknowledgements
We would like to thank Niclas Setterbald (Confocal Imagery Department (IFR 105) of the Institut Universitaire d'Hematologie, Hopital Saint Louis, Paris, France) for technical help. This work was supported by the “L'Association pour la Recherche sur le Cancer” (ARC), “Commisariat a l'Energie Atomique” (CEA) and “Fondo de Investigación Sanitaria” (FIS-CM06/00113).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Intact HLA-G-containing membrane patches transfer to monocytes and T cells and this transfer is cell-to-cell contact dependent. (PDF 3728 kb)
Rights and permissions
About this article
Cite this article
Alegre, E., HoWangYin, KY., Favier, B. et al. Membrane redistributions through multi-intercellular exchanges and serial trogocytosis. Cell Res 20, 1239–1251 (2010). https://doi.org/10.1038/cr.2010.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2010.136
Keywords
This article is cited by
-
Immunomodulating functions of human leukocyte antigen-G and its role in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2021)
-
Trogocytic intercellular membrane exchanges among hematological tumors
Journal of Hematology & Oncology (2015)