Abstract
This study examined the signaling events induced by shikonin that lead to the induction of apoptosis in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells (e.g., K562, LAMA84). Treatment of K562 cells with shikonin (e.g., 0.5 μM) resulted in profound induction of apoptosis accompanied by rapid generation of reactive oxygen species (ROS), striking activation of c-Jun-N-terminal kinase (JNK) and p38, marked release of the mitochondrial proteins cytochrome c and Smac/DIABLO, activation of caspase-9 and -3, and cleavage of PARP. Scavenging of ROS completely blocked all of the above-mentioned events (i.e., JNK and p38 phosphorylation, cytochrome c and Smac/DIABLO release, caspase and PARP cleavage, as well as the induction of apoptosis) following shikonin treatment. Inhibition of JNK and knock-down of JNK1 significantly attenuated cytochrome c release, caspase cleavage and apoptosis, but did not affect shikonin-mediated ROS production. Additionally, inhibition of caspase activation completely blocked shikonin-induced apoptosis, but did not appreciably modify shikonin-mediated cytochrome c release or ROS generation. Altogether, these findings demonstrate that shikonin-induced oxidative injury operates at a proximal point in apoptotic signaling cascades, and subsequently activates the stress-related JNK pathway, triggers mitochondrial dysfunction, cytochrome c release, and caspase activation, and leads to apoptosis. Our data also suggest that shikonin may be a promising agent for the treatment of CML, as a generator of ROS.
Similar content being viewed by others
Introduction
Shikonin (C16H16O5) is a natural naphthoquinone derivative compound that is present in the root tissues of the traditional Chinese medical herb Lithospermum erythrorhizon. This herb is found throughout China, including in Southern Yun Nan, Northern Dong Bei, Western Xin Jiang and Tibet. Lithospermum erythrorhizon was first recorded in a 2000-year-old Chinese medical material dictionary “Materia Medica of Deity of Agriculture”, also called “Shen Nong Ben Cao Jing” 1. This dictionary was compiled around the time of the Qin (225 BC–206 BC) and Han (206 BC–220 AD) dynasties. In addition, Lithospermum erythrorhizon was described as a herbal medicine in Shi-Zhen Li's (1518–1593) “Compendium of Materia Medica”, which is also called “Ben Cao Gang Mu” 2, and was written during the Ming dynasty (1368–1644). Lithospermum erythrorhizon has been broadly applied as a traditional Chinese medicine (TCM) for thousands of years in China to treat burns and to promote wound healing; it also has anti-bacterial and anti-inflammatory properties. Later, it was found that shikonins, including the individual compounds shikonin, isobutyryl shikonin, acetyl shikonin, dimethylacryl shikonin and isovaleryl shikonin, are responsible for the anti-bacterial and anti-inflammatory activities of this TCM 3, 4, 5, 6.
Recently, it was reported that shikonin induces apoptosis of various cancer cells and performs anti-cancer activities 7, 8, 9, 10. Studies suggest that the anti-cancer activities of shikonin derivatives may be attributed to their ability to down-regulate anti-apoptotic Bcl-2 family members, such as Bcl-2 and Bcl-xL, generate reactive oxygen species (ROS), activate caspases 11, 12, inhibit EGFR phosphorylation 13 and arrest the cell cycle through p53 upregulation 14. However, the precise mechanisms employed by shikonin to repress cancer growth remain to be defined.
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder characterized by increased proliferation of granulocytic cells without loss in their capacity to differentiate. This abnormality results from a reciprocal translocation between the abl oncogene on the long arm of chromosome 9 and the bcr region on the long arm of chromosome 22. This translocation, designated Bcr/Abl 9, results in lengthening of an arm of chromosome 9 and shortening of an arm of chromosome 22. The latter chromosome is termed the Philadelphia chromosome (Ph1), named after the city where it was discovered. The resulting bcr-abl fusion gene encodes a chimeric protein with strong tyrosine kinase activity. This cytogenetic hallmark (bcr/abl) is present in more than 90% of CML cases 15, 16. CML is the first described malignancy associated with a gene translocation. Presence of the Bcr/Abl tyrosine kinase renders CML resistant to traditional therapeutic approaches, which makes Bcr/Abl an important therapeutic target. The first Bcr/Abl tyrosine kinase inhibitor, STI571 (Gleevec, imatinib mesylate) 17, was recently presented as a revolutionary approach to CML treatment. STI571 is now the first-choice treatment for all newly diagnosed CML patients. Oral administration of STI571 results in a clinical response in more than 90% of CML patients. Importantly, the striking initial efficacy of this drug has been overshadowed by the development of clinical resistance. The mechanisms of resistance to STI571 include Bcr/Abl amplification, mutations in the Bcr/Abl kinase domain and diminished drug uptake, among others 18, 19. Thus, it is important to search for additional approaches to treat this malignancy.
Apoptosis was originally defined by Kerr 20 as the orderly and characteristic sequence of structural changes resulting in the programmed death of the cell. Apoptosis is cell suicide achieved through well-characterized morphological and biochemical changes. Apoptosis appears to be a common mechanism of biological activity for cytotoxic agents employed in chemotherapy. There are two well-studied pathways that result in apoptosis: the cell surface death receptor pathway and the mitochondria-initiated pathway. In the cell surface death receptor pathway, death receptors detect the presence of extracellular death signals and rapidly ignite the cell's intrinsic apoptosis machinery 21, 22, 23. These death receptors include CD95, TNFR1, avian CAR1, DR3, DR4 and DR5. In the mitochondria-initiated pathway, caspase activation is triggered by formation of the multimeric Apaf-1/cytochrome c complex, which recruits and activates procaspase-9. Activated caspase-9 cleaves and activates downstream caspases, such as caspase-3, -6 and -7. The release of mitochondrial cytochrome c is regulated by Bcl-2 family proteins, including the anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl-1 and the pro-apoptotic proteins Bax, Bad, Bak and Bid 24, 25, 26, 27, 28, 29.
ROS are continually generated and eliminated in biological systems. They play important roles in a variety of normal biochemical functions and abnormal pathological processes. Excessive production of ROS in the cell is known to induce apoptosis 30, 31. The ability of ROS to inflict severe cellular damage and cause cell death has been exploited as an approach to kill cancer cells. ROS generation has also been shown to play an important role in apoptosis induced by treatment with cisplatin, bleomycin, UV irradiation and Bortezomib (PS341), a proteosome inhibitor recently approved by the FDA for the treatment of multiple myeloma 32.
c-Jun-N-terminal kinase (JNK) is a member of the mitogen-activated protein (MAP) kinase family. Increasing evidence indicates a crucial role of JNK in mitochondrial dysfunction and the subsequent initiation of apoptosis. More specifically, apoptosis is achieved by JNK-initiated release of apoptosis-regulating factors, such as cytochrome c and Smac/DIABLO 33, 34, JNK-mediated inactivation of anti-apoptotic proteins, JNK-mediated activation of pro-apoptotic Bcl-2 family proteins 35, 36 and JNK-induced expression of pro-apoptotic proteins, such as Bim 37.
Although the efficacy of shikonin and its derivatives has been broadly studied, their precise mode of action and the molecular basis of their anti-cancer activities remain to be elucidated. Here, we report that a shikonin induces apoptosis of CML cells by the following processes: ROS generation, JNK/c-JUN activation, mitochondrial dysfunction, cytochrome c release and caspase activation. The present data also suggest that shikonin may serve as a novel agent for the treatment of CML as it induces excessive ROS production.
Results and Discussion
Shikonin induces apoptosis of CML cells in a dose- and time-dependent manner
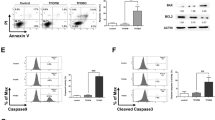
Shikonin, the chemical structure 3, 11 of which is shown in Figure 1A, is a natural naphthoquinone-derived compound present in the root tissues of the Chinese herb Lithospermum erythrorhizon. Shikonin induces apoptosis in various cancer cell lines and performs anti-tumor activity in vivo 7, 8, 9, 10. To determine whether shikonin mediates apoptosis of CML cells, Bcr/Abl-positive K562 cells were treated with 0.5 μM shikonin for 24 h. A high percentage (∼ 85%) of cells underwent apoptosis, as detected by Annexin V/propidium iodide (PI) analysis (Figure 1B). To further explore shikonin-induced apoptosis, K562 cells were exposed to increasing concentrations of shikonin for varying lengths of time, as indicated (Figure 1C). A significant percentage of cells (∼ 30%) underwent apoptosis following exposure to 300 nM shikonin for 16 h. The percentage of apoptotic cells dramatically increased with increasing concentrations of shikonin, from 200 to 500 nM, when incubated for 12-16 h. A parallel study was performed in another Bcr/Abl-positive cell line, LAMA 84 (Figure 1D). A significant percentage of these cells (∼ 40%) underwent apoptosis following treatment with 200 nM shikonin for 16 h. Comparatively, LAMA 84 cells were more sensitive to shikonin than K562 cells.
Apoptosis induced by shikonin treatment of CML cells. (A) The chemical structure of shikonin. (B) After K562 cells were treated with 0.5 μM shikonin for 24 h, the percentage of cells undergoing apoptosis was determined by flow cytometry following Annexin V/PI staining. Cells that were positive for Annexin V but not PI, which are present in the lower right quadrant, are early apoptotic cells. Cells that were both Annexin V- and PI-positive, which are present in the upper right quadrant, are late apoptotic cells. After K562 (C) and LAMA 84 (D) cells were exposed to increasing concentrations of shikonin for the designed time intervals, apoptosis was revealed by DAPI staining.
Shikonin-induced apoptosis of CML cells involves both mitochondria and caspases
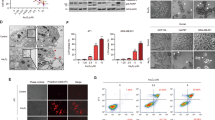
Shikonin has been reported to induce apoptosis through a mitochondria- and caspase-mediated pathway in human colorectal carcinoma cells 11, and through a mitochondria-independent pathway in human SK-Hep-1 hepatoma cells 12. To investigate whether mitochondria are involved in shikonin-induced apoptosis of CML cells, the percentage of cells that exhibit “low” 3,3-dihexyloxacarbocynine (DiOC6) uptake, which reflects a loss of mitochondrial membrane potential (Δψm), was examined using the DiOC6 staining assay after shikonin treatment. The data obtained from flow cytometry experiments demonstrate that shikonin mediates a mitochondrial permeability transition (loss of Δψm) in K562 cells (Figure 2A), which suggests that mitochondria are involved in shikonin-induced apoptosis. To determine the contribution of caspases to this process, K562 cells were treated with shikonin in the absence or presence of a broad-spectrum caspase inhibitor, BOC-fmk, for 24 h. Shikonin-induced apoptosis was completely blocked by BOC-fmk treatment (Figure 2B). Furthermore, western blotting analysis indicated that shikonin treatment resulted in a profound mitochondrial release of cytochrome c and Smac/DIABLO, cleavage of myeloid cell leukemia-1 (Mcl-1, an anti-apoptotic protein in the Bcl-2 family), caspase-3 and -9 and PARP. The release of Smac/DIABLO and cleavage of Mcl-1, caspase-3 and -9 and PARP were blocked by BOC-fmk, while the release of cytochrome c was not affected by BOC-fmk (Figure 2C). Altogether, these results indicate that cytochrome c release, which is an indicator of mitochondrial injury, is upstream of caspase activation and that Mcl-1 attenuation is secondary to caspase activation, as it is a caspase-mediated event. In this case, cleavage of Mcl-1 may amplify the extent of apoptosis induced by shikonin. This hypothesis is based on previous studies that demonstrated that caspase-mediated cleavage of Mcl-1 inhibits its anti-apoptotic function 38. In addition, we noted that BOC-fmk did not completely block cleavage of caspase-9. This is expected since this caspase is autocatalytic. Together, these results suggest that shikonin-induced apoptosis in K562 cells is mediated by a pathway that involves mitochondria injury, cytochrome c release and caspase activation.
Apoptosis is induced by shikonin through a mitochondria and caspase-mediated pathway in CML cells. (A) After K562 cells were treated with the indicated concentrations of shikonin for 24 h, DiOC6 uptake was examined by flow cytometry. (B) After K562 cells were treated with 0.5 μM shikonin in the absence or presence of 50 μM BOC-fmk for 24 h, apoptosis was quantified under a microscope by DAPI staining. (C) Western blot analyses of Bcl-xL, Mcl-1, caspase-9, cleaved caspase-3 and PARP from whole cell lysates were performed using treatment regimens similar to those described in (B). In addition, S-100 fractions were obtained and submitted to Western blot analyses of cytochrome c (cyto c) and Smac/DIABLO. Actin served as loading control. CF: cleaved fragment. After repeating the experiment three times, similar results/patterns were obtained. Thus, representative Western blots are shown in the figure.
ROS trigger multiple signaling cascades involved in shikonin-induced apoptosis
A previous study reported that shikonin induces ROS formation, but that it does not induce a mitochondrial permeability transition in the course of inducing apoptosis of human SK-Hep-1 hepatoma cells 12. However, the results shown in Figure 2A demonstrate that mitochondria are involved in shikonin-induced apoptosis of K562 cells. In order to test whether shikonin leads to ROS generation in CML cells, K562 cells were treated with shikonin, as indicated in Figure 3A. Rapid generation of ROS was detected at 1 h following shikonin treatment. Importantly, ROS production was markedly blocked by LNAC treatment (L-N-acetylcysteine, free radical scavenger) (P< 0.001) (Figure 3A). Consequently, apoptosis induced by shikonin was also completely abolished by LNAC treatment (Figure 3B). It is known that ROS activates multiple signaling pathways, including MAPK and Akt signal transduction cascades 30. Western blot analysis was employed to evaluate the activity of MAPK and Akt signal transduction pathways after shikonin treatment (Figure 3C). The results show that shikonin-induced ROS generation was followed by striking increases in the levels of phospho-JNK, phospho-c-JUN and phospho-p38, a significant decrease in the level of phospho-Erk1 (44 kDa), as well as a profound release of cytochrome c, and cleavage of caspase-3 and -9 and PARP. LNAC treatment completely reversed JNK and p38 phosphorylation, cytochrome c release and caspase cleavage, suggesting that ROS generation is upstream of the examined mediators. Moreover, LNAC completely blocked shikonin-mediated Smac/DIABLO release (data not shown). However, there was no appreciable modification of phosphorylation of Erk2 (42 kDa), Akt or GSK 3 after LNAC treatment.
Shikonin-induced ROS generation in CML cells. (A) K562 cells were treated with 0.5 μM shikonin in the absence or presence of 10 mM LNAC for 1 h and ROS generation was examined by flow cytometry. * P < 0.001. (B) After treatment for 24 h, apoptosis was quantified by DAPI staining. (C) Western blot analyses of phospho-Erk1/2, phospho-JNK, phospho-c-JUN, phospho-p38, phospho-Akt, phospho-GSK 3, caspase-9, cleaved caspase-3 and PARP from the whole cell lysates were performed after shikonin treatment for 24 h of p-JNK (top, middle and bottom bands in the blots are JNK2, JNK3 and JNK1, respectively) and p-GSK3 (top and bottom bands are GSK3α and GSK3β, respectively). In addition, S-100 fractions were isolated for Western blot analysis of cytochrome c (cyto c). (D) ROS generation was examined by flow cytometry after K562 cells were treated with 0.5 μM shikonin in the absence or presence of 30 μM SP600125, 10 μM SB203580 and 50 mM BOC-fmk for 1 h. CF: cleaved fragment. After repeating the experiment three times, similar results/patterns were obtained. Thus, representative Western blots are shown in the figure.
If ROS generation is the proximal event of JNK and p38 activation in shikonin-treated K562 cells, inhibition of JNK, p38 and caspase activity would not influence ROS generation. To test this hypothesis, K562 cells were exposed to shikonin in the absence or presence of JNK, p38 and caspase inhibitors, independently. As shown in Figure 3D, ROS generation was not appreciably modified after addition of the inhibitors (SP600125, a specific JNK inhibitor; SB203580, a specific p38 inhibitor; and BOC-fmk, a pan-caspase inhibitor). These results are consistent with the prediction that ROS generation is the initial event and that it precedes activation of the MAPK and Akt signaling cascades. Results from parallel experiments performed in LAMA84 cells showed that a significant amount of ROS (e.g., 9-fold increase) was generated after only 1 h of exposure to shikonin, which was blocked by LNAC (data not shown).
JNK activation contributes to shikonin-induced apoptosis of CML cells
The preceding results indicated that shikonin activated JNK, c-JUN and p38 signaling cascades (Figure 3C). We next tested the functional roles of these signals in drug-induced apoptosis. K562 cells were treated with shikonin in the absence or presence of pharmacological inhibitors of JNK and p38. A time-course study showed that JNK activation persists after treatment with 0.5 μM shikonin (Figure 4A). SP600125, a specific JNK inhibitor, markedly blocked shikonin-induced apoptosis (P< 0.001). However, SB203580, a specific p38 inhibitor, did not appreciably modify drug-induced apoptosis (Figure 4B). Western blotting analysis showed that treatment with SP600125 markedly blocked shikonin-induced c-JUN phosphorylation, cytochrome c release, and cleavage of caspase-3 and -9 and PARP, while SB203580 had no effect on any of these events (Figure 4C).
JNK activation by shikonin contributed to drug-induced apoptosis of CML cells. (A) The time-course study of JNK activation after 0.5 μM shikonin treatment in K562 cells. (B) After K562 cells were treated with shikonin in the absence or presence of 30 μM SP600125, or the absence or presence of 10 μM SB203580, for 24 h apoptosis was examined by DAPI staining. * P < 0.001. (C) Western blot analyses of phospho-c-JUN, caspase-9, cleaved caspase-3 and PARP from whole cell lysates were performed after treatment for 24 h. In addition, S-100 fractions were isolated for Western blot analysis of cytochrome c. (D) After K562 cells were transfected with JNK1-siRNA for 48 h, followed by a 24 h treatment with 0.5 μM shikonin, Western blot analyses of the indicated proteins were performed, and apoptosis was evaluated by DAPI staining (E). CF: cleaved fragment, cyto c: cytochrome c. After repeating the experiments three times, similar results/patterns were obtained. Thus, representative Western blots are shown in the figure.
In order to determine whether JNK activation contributes to shikonin-induced apoptosis, K562 cells were transfected with a JNK1 short interfering RNA (siRNA) and then treated with shikonin. Western blotting analysis indicated that knock-down of JNK1 markedly blocked cytochrome c release and PARP cleavage (Figure 4D) as well as the induction of apoptosis (Figure 4E). In addition, decreased JNK1 activity, achieved by JNK siRNA-mediated knock-down or JNK inhibition by SP600125, did not affect shikonin-mediated ROS generation (data not shown). Together, these data suggest that activation of JNK, and not p38, plays a functional role in shikonin-induced apoptosis. These results also indicate that JNK activation is upstream of cytochrome c release, caspase activation and PARP cleavage.
Putative model of signaling cascades in shikonin-induced apoptosis of CML cells
The above results suggest a model in which shikonin induces apoptosis by inducing production of ROS, which subsequently leads to activation of JNK, release of cytochrome c, and cleavage of caspases and PARP. Blockage of ROS generation, JNK activation and caspase activation affected the corresponding downstream cascades (Figure 5). ROS generation by shikonin represents the proximal and initiating event in drug-induced apoptosis.
A putative model of the hierarchical signaling events leading to apoptosis of CML cells following treatment with shikonin. Shikonin induces the generation of ROS, activation of JNK, release of cytochrome c, and cleavage of caspases and PARP. ROS generation by shikonin is the proximal event that triggers multiple signaling cascades in drug-induced apoptosis.
In summary, our results indicate that apoptosis induced by shikonin treatment of CML cells is caused by the rapid generation of ROS, which triggers JNK phosphorylation, mitochondrial injury and caspase activation. ROS generation and JNK activation play critical roles in shikonin-induced apoptosis. This result also suggests that shikonin may serve as a novel agent for CML treatment by inducing excessive production of ROS.
Materials and Methods
Cells
The K562 cell line was purchased from American Type Culture Collection, Rockville, MD. The LAMA84 cell line was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Sub-confluent, logarithmically growing cells were placed in sterile plastic T-flasks, to which the designated drugs were added. The flasks were then incubated in a humidified incubator with 5% CO2 at 37 °C for various lengths of time, as indicated.
Materials
Shikonin was obtained from Shanghai TCI Chemical Co. (Shanghai, China); this chemical had a purity of >97%. BOC-D-fmk was purchased from Enzyme System Products (Livermore, CA). LNAC, DiOC6, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), PI and SP600124 were obtained from Sigma Chemical Co. (Beijing, China).
Assessment of apoptosis
Following drug treatment, apoptotic cells were detected by DAPI staining, which allowed for identification of apoptotic nuclear changes as described previously 39. Briefly, 1 × 105 cells were harvested and washed with phosphate-buffered saline (PBS) and then fixed with 1% glutaraldehyde at room temperature for 30 min. After washing with PBS, cells were re-suspended in 20 μl of PBS and mixed with 5 μl of a 10 μg/ml stock solution of DAPI. Cell suspensions were mounted on slides. Cells were visualized under microscope with a 360-370 nm excitation light and 420-460 nm emission filter; apoptotic cells appeared with characteristic condensed and fragmented nuclei.
Annexin V/PI staining
To confirm the results of morphological analysis, Annexin V/PI staining was employed. For Annexin V/PI assays, cells were stained with Annexin V-FITC and PI and then monitored for apoptosis by flow cytometry in accordance with the manufacturer's protocol (BD PharMingen, San Diego, CA, USA). Briefly, 0.5 ×106 cells were washed twice with PBS and stained with 5 μl of Annexin V-FITC and 10 μl of PI (5 μg/ml) in 1× binding buffer (10 mM HEPES, pH 7.4, 140 mM NaOH, 2.5 mM CaCl2) for 15 min at room temperature in the dark. Apoptotic cells were quantified using a FACScan cytofluorometer. Both early apoptotic (Annexin V-positive, PI-negative) and late apoptotic (double positive of Annexin V and PI) cells were detected.
Assessment of mitochondrial membrane potential (Δψm)
Mitochondrial membrane potential was monitored using the fluorescent dye DiOC6. For each condition, 4 × 105 cells were incubated for 30 min at 37 °C in 1 ml of 40 nM DiOC6 and subsequently analyzed using a FACScan cytofluorometer with excitation and emission settings of 488 and 525 nm, respectively. The percentage of cells exhibiting a low level of DiOC6 uptake exhibited a loss of mitochondrial membrane potential.
Detection of ROS
Generation of ROS was assessed using fluorescent probe dihydroethidium (DHE) staining, as was previously described 40. In the presence of superoxide (O2−), DHE is oxidized to fluorescent products, which are monitored by flow cytometry. Briefly, cells were incubated with 20 μM DHE in tissue culture medium for 30 min at 37°C, and then washed, re-suspended in PBS, and subsequently monitored by flow cytometry.
Western blot analysis
Following drug treatment, cell lysates were prepared and Western blot analysis was performed with primary antibodies, as described previously 40. The following antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, USA): caspase 9, cleaved caspase 3, cytochrome c, phospho-ERK1/2, phospho-JNK, phospho-c-JUN (Ser73), phospho-Akt, phospho-p38 and actin. To ensure equivalent loading and transfer, all blots that had been probed for proteins other than actin were stripped and re-probed with an antibody against actin. The western blots shown in the figures are representative of three independent experiments unless otherwise indicated.
Assessment of cytochrome c release from mitochondria
Following drug treatment, the release of cytochrome c from mitochondria was analyzed by a selective digitonin permeabilization method. Briefly, 4 × 106 cells per condition were re-suspended in 50 μl of permeabilization buffer (containing 75 mM NaCl, 8 mM Na2PO4, 1 mM NaH2PO4, pH 7.4, 250 mM sucrose (added fresh before use), 1 mM EDTA, 700 μg/ml digitonin) (final concentration of digitonin 35 μg/4 × 106 cells). Cells were incubated in the above permeabilization buffer at room temperature for 1 min, after which the pellet was removed by centrifugation for 3 min at 13 000 × g, and the supernatant containing cytochrome c protein was obtained.
siRNA-mediated knock-down
A JNK1-specific siRNA (5′-GCAGAAGCAAACGTGAC AACA-3′) and a negative control siRNA (Catalog# AM4635) were purchased from Ambion (Austin, TX, USA). Cells were transfected with siRNAs at a final concentration of 30 nM using siPORT™ NeoFX™ Transfection Agent, following the manufacturer's protocol.
Statistical analysis
All of the data are expressed as mean ± SD of three individual experiments. Differences between groups were determined using the Student's t test for unpaired observations. P < 0.05 was considered significant.
References
Shen N, ed. Shen Nong Ben Cao Jing. Lanzhou: Lanzhou University Press, 2004.
Li SZ, ed. Ben Cao Gang Mu. Chongqing: Chongqing University Press, 1994.
Staniforth V, Wang SY, Shyur LF, et al. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibits the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem 2004; 279:5877–5885.
Shen CC, Syu JJ, Li YY, et al. Antimicrobial activities of naphthazarins from Arnebia euchroma. J Nat Prod 2002; 65:1857–1862.
Singh B, Sharma MK, Meghwal PR, et al. Anti-inflammatory activity of shikonin derivatives from Arnebia hispidissima. Phytomedicine 2003; 10:375–380.
Chen X, Oppenheim J, Howard OM . Shikonin, a component of anti-inflammatory Chinese herbal medicine, selectively blocks chemokine binding to CC chemokine receptor-1. Int Immunopharmacol 2001; 1:229–236.
Sankawa U, Ebizuka Y, Miyazaki T, et al. Antitumor activity of shikonin and its derivatives. Chem Pharm Bull 1977; 25:2392–2395.
Sankawa U, Otsuka H, Kataoka Y, et al. Antitumor activity of shikonin, alkannin and their derivatives. II. X-ray analysis of cyclo-alkannin leucoacetate, tautomerism of alkannin and cyclo-alkannin and antitumor activity of alkannin derivatives. Chem Pharm Bull 1981; 29:116–122.
Gao D, Hiromura M, Yasui H, et al. Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol Pharm Bull 2002; 25:827–832.
Hashimoto S, Xu M, Masuda Y, et al. β-Hydroxyisovalerylshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J Biochem 1999; 125:17–23.
Hsu PC, Huang YT, Tsai ML, et al. Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem 2004; 52:6330–6337.
Chen CH, Chern CL, Lin CC, et al. Involvement of reactive oxygen species, but not mitochondrial permeability transition in the apoptotic induction of human SK-Hep-1 hepatoma cells by shikonin. Planta Med 2003; 69:1119–1124.
Singh F, Gao D, Lebwohl MG, et al. Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett 2003; 200:115–121.
Wu Z, Wu L, Li L, et al. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J Pharmacol Sci 2004; 94:166–176.
Rowley JD . A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973; 243:290–293.
Bartram CR, Klein A, Hagemeijer A, et al. Translocation of c-abl oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 1983; 306:277–280.
Druker BJ . Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene 2002; 21:8541–8546.
Gorre ME, Mohammed M, Ellwood K . Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293:876–880.
Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002; 2:117–125.
Kerr JFR, Wyllie AH, Currie AR, et al. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer 1972; 26:239–257.
Ashkenazi A, Dixit VM . Death receptors: signaling and modulation. Science 1998; 281:1305–1308.
Smith CA, Farrah T, Goodwin RG . The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 1994; 76:959–962.
Budihardjo I, Oliver H, Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15:269–290.
Wang X . The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15:2922–2933.
Liu X, Kim CN, Yang J, et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996; 86:147–157.
Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275:1129–1132.
Gross A, McDonnell JM, Korsmeyer SJ . BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999; 13:1899–1911.
Du C, Fang M, Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102:33–42.
Craig RW . MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia 2002; 16:444–454.
Martindale JL, Holbrook NJ . Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 2002; 192:1–15.
Sastre J, Pallardo FV, Vina J . Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 2000; 49:427–435.
Pelicano H, Carney D, Huang P . ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 2004; 7:97–110.
Kanda H, Miura M . Regulatory roles of JNK in programmed cell death. J Biochem (Tokyo) 2004; 136:1–6.
Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 2000; 288:870–874.
Kharbanda S, Saxena S, Yoshida K, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA damage. J Biol Chem 2000; 275:322–327.
Donovan N, Becker EB, Konishi Y . JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem 2002; 277:40944–40949.
Putcha GV, Le S, Frank S, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 2003; 38:899–914.
Herrant M, Jacquel A, Marchetti S, et al. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene 2004; 23:7863–7873.
Yu CR, Mao X, Li WX . Inhibition of the PI3K pathway sensitizes fludarabine-induced apoptosis in human leukemic cells through an inactivation of MAPK-dependent pathway. Biochem Biophys Res Commun 2005; 331:391–397.
Bass DA, Parce JW, Dechatelet LR . Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 1983; 130:1910–1917.
Acknowledgements
This work was supported by grants from the National High Technology Research and Development Program of China (863 Program) (2006AA02A306), the National Natural Science Foundation of China (No. 39900082) and the PhD Program Foundation of Ministry of Education of China (No. 204090188). We thank Dr Courtney M Heney (Massachusetts General Hospital, Harvard University) for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, X., Rong Yu, C., Hua Li, W. et al. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res 18, 879–888 (2008). https://doi.org/10.1038/cr.2008.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2008.86
Keywords
This article is cited by
-
NR4A1 enhances MKP7 expression to diminish JNK activation induced by ROS or ER-stress in pancreatic β cells for surviving
Cell Death Discovery (2021)
-
The natural alkaloid Jerantinine B has activity in acute myeloid leukemia cells through a mechanism involving c-Jun
BMC Cancer (2020)
-
Combination therapy of BCR-ABL-positive B cell acute lymphoblastic leukemia by tyrosine kinase inhibitor dasatinib and c-JUN N-terminal kinase inhibition
Journal of Hematology & Oncology (2020)
-
Insights into the mechanism of Arnebia euchroma on leukemia via network pharmacology approach
BMC Complementary Medicine and Therapies (2020)
-
Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells
Journal of Experimental & Clinical Cancer Research (2019)