Abstract
Mitogen-activated protein kinases (MAPKs) are involved in the regulation of plant growth, development and responses to a wide variety of stimuli. In a conditional gain-of-function transgenic system, the activation of AtMEK5, a MAPK kinase, can in turn activate endogenous AtMAPK3 and AtMAPK6, and can lead to a striking increase in ethylene production and induce hypersensitive response (HR)-like cell death in Arabidopsis. However, the role of the increased ethylene production in regulating this HR-like cell death remains unknown. Using Arabidopsis transgenic plants that express AtMEK5DD, an active mutant of AtMEK5 that is under the control of a steroid-inducible promoter, we tested the contribution of ethylene to cell death. We found that ethylene biosynthesis occurs before cell death. Cell death was delayed by inhibiting AtMEK5-induced ethylene production using inhibitors of ACC-synthases, ACC-oxidases or ethylene receptors. In the mutants AtMEK5DD/etr1-1 and AtMEK5DD/ein2-1, both of which showed insensitivity to ethylene, the expression of AtMEK5DD protein, activity of AtMAPK3 and AtMAPK6, and ethylene production were the same as those seen in AtMEK5DD transgenic plants, but cell death was also delayed. These data suggest that ethylene signaling perception is required to accelerate cell death that is induced by AtMEK5 activation.
Similar content being viewed by others
Introduction
Cell death is a fundamental biological process in plant development and is involved in responses to diverse stimuli, including biotic and abiotic stresses 1, 2. To prevent pathogen invasion, plants have developed a complex array of defense responses that include generation of reactive oxygen species (ROS), activation of defense genes, production of phytoalexins, and rapid cell death, which is also known as the hypersensitive response (HR) 3, 4, 5, 6, 7, 8. HR-like cell death confines the pathogen by stopping its spread from the site of the attempted infection. The results obtained from kinase activity assays and pharmacological studies that use specific inhibitors of protein kinases and phosphatases have implicated protein phosphorylation and dephosphorylation in the induction of defense responses in plants 9, 10, 11. Mitogen-activated protein kinase (MAPK) cascades are evolutionarily conserved signaling modules that transduce the extracellular stimuli into intracellular signals to regulate a wide variety of cellular processes in all eukaryotes 12, 13, 14. Pathogens and pathogen-derived elicitors can activate MAPK and induce HR-like cell death, while kinase activity inhibitors block HR-like cell death. Such evidence suggests that the activation of MAPK cascades is involved in the signaling pathways that lead to HR-like cell death 15, 16, 17, 18.
MAPK cascades are composed of three functional interlinked protein kinases: MAPKs, MAPK kinases (MAPKKs or MEKs) and MAPKK kinases (MAPKKKs or MEKKs). MAPKKKs can be activated by different mechanisms, such as phosphorylation or interaction with G-protein or receptors. MAPKKKs activate MAPKK through the phosphorylation of its two serine/threonine residues in the S/T-X3-5-S/T motif. MAPKKs are dual-specificity kinases that activate MAPK through the phosphorylation of threonine and tyrosine residues in the T-X-Y motif. Activated MAPKs may be translocated to the nucleus to phosphorylate and thereby regulate the activity of specific transcription factor(s). Alternatively, activated MAPKs can remain in the cytoplasm to phosphorylate cytoskeleton-associated proteins or regulatory enzymes and to initiate cellular responses 19.
The results of gain-of-function studies have revealed that expression of an active mutant of NtMEK2, a MAPKK in tobacco, induces HR-like cell death that is preceded by the activation of endogenous WIPK, Ntf4 and SIPK, all of which are downstream MAPKs of NtMEK2 20, 21. Overexpression of SIPK or Ntf4 in tobacco leaves leads to increased activity of SIPK or Ntf4 that is sufficient to induce HR-like cell death 21, 22. Although the overexpression of WIPK alone fails to elicit cell death, co-expression of WIPK with the active mutant of NtMEK2 leads to accelerated HR-like cell death 17. In addition, by using the PVX-induced gene silencing technique, Jin and colleagues reported that the suppression of all three known components in the NtMEK2–SIPK/WIPK pathway attenuated N gene-mediated TMV resistance, possibly by regulating HR-like cell death 23. Based on phylogenetic analysis, Arabidopsis AtMEK4 and AtMEK5 and tomato LeMKK2 are very similar to tobacco NtMEK2, and they belong to MAPKK group C 24, 25, 26. The expression of active mutants of LeMKK2, AtMEK4, or AtMEK5 in transgenic plants causes HR-like cell death and activates their respective downstream MAPKs: LeMPK2 and LeMPK3, AtMAPK6, and AtMAPK3 24, 25. LeMPK2 and AtMAPK6 are very similar to SIPK, and LeMPK3 and AtMAPK3 are very similar to WIPK. Overexpression of LeMKK4, which is not related phylogenetically to LeMKK2 and belongs to the MAPKK group D, also elicits HR-like cell death and activates LeMPK2 and LeMPK3 in tomato 25. We have observed that the activation of AtMEK7 and AtMEK9, which also belong to the MAPKK group D, can induce HR-like cell death and activate AtMAPK3 and AtMPAK6 in transient transformed tobacco plants and permanent transgenic Arabidopsis plants (our unpublished data). Collectively, these results indicate that MAPK cascades are involved in the regulation of HR-like cell death in plants.
Ethylene, a gaseous phytohormone, has important roles in regulating plant growth and development, and participates in plant responses to both biotic and abiotic stresses 27, 28. There are two crucial steps in the control of ethylene biosynthesis. The first is the rate-limiting conversion of S-adenosyl-L-methionine (SAM) to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) 29. The second is the oxidative cleavage of ACC by ACC oxidase (ACO) to form ethylene, CO2 and cyanide 29, 30. The acceleration of ethylene biosynthesis in response to environment stresses and endogenous cues is often related to the increased activity of ACS that is caused by the post-transcriptional regulation and activation of ACS and ACO 28. In tomato cells, treatment with inhibitors of protein kinases prevented the increase of ACS activity that is induced by a fungal elicitor, whereas treatment with a phosphatase inhibitor stimulated ACS activity in the absence of the elicitor 31. Ethylene production in NN tobacco plants that were infected with TMV correlated with SIPK/WIPK activation and overexpression of ACSs and ACOs 18, 32, 33. Expression of the active mutant of NtMEK2 induced rapid activation of SIPK/WIPK, accelerated ethylene biosynthesis and caused HR-like cell death in transgenic tobacco plants 18, 20. Recently, Liu and Zhang reported that ethylene biosynthesis, which was induced by the activation of NtMEK2, was the result of phosphorylation of ACS6 by AtMAPK6 34. These results suggest that protein phosphorylation and dephosphorylation are involved in the regulation of stress-induced ethylene biosynthesis.
It has been established that the activation of NtMEK2 and its Arabidopsis orthologs (AtMEK4 and AtMEK5) are involved in ethylene biosynthesis, H2O2 generation and HR-like cell death 18, 24, 34. However, the relationship between ethylene and this HR-like cell death is not yet fully understood. In this report, we present evidence, obtained from transgenic Arabidopsis plants that carry the steroid-inducible promoter controlled AtMEK5DD, for the role of ethylene in regulating HR-like cell death. We found that induction of AtMEK5DD, an active mutant of AtMEK5, led to rapid activation of endogenous AtMAPK3 and AtMAPK6, a dramatic increase in ethylene production and ultimately HR-like cell death. Cell death was delayed by using inhibitors of ethylene biosynthesis and by blocking ethylene perception with ethylene receptor antagonist or ethylene receptor mutants. These results suggest that ethylene and its signaling pathways have important roles in AtMEK5-induced HR-like cell death.
Results
Activation of AtMEK5 induces ethylene biosynthesis and cell death
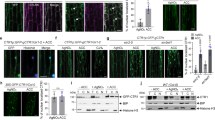
In a previous study, we showed that the expression of AtMEK5DD, an active form of AtMEK5, could activate downstream endogenous AtMAPK3 and AtMAPK6 and cause HR-like cell death in transgenic Arabidopsis plants 24. More recently, several investigators have reported that activation of NtMEK2, as well as its orthologs in Arabidopsis (AtMEK4 and AtMEK5), can accelerate ethylene biosynthesis in transgenic plants 18, 34. In this instance, ethylene production was the result of phosphorylation of ACS6 by activated AtMAPK6 and the resultant accumulation of ACS protein in Arabidopsis 34. However, the molecular mechanisms that link activation of the MAPK cascade and induction of HR-like cell death remain to be elucidated. In this study, we used transgenic Arabidopsis plants that carried AtMEK5DD (the mutated gene that encodes the active AtMEK5 protein), AtMEK5KR (the mutated gene that encodes the inactive AtMEK5 protein) and AtMEK5WT (the wild-type gene that encodes the AtMEK5 protein) under control of the steroid-induced promoter to better understand the relationship between ethylene production and HR-like cell death 24. We used the Evans blue staining method to detect cell death. Small areas of cell death were detected on the leaf edge of AtMEK5DD plants 24 h after Dexamethasone (DEX) treatment and the whole leaf died after 36 h after DEX treatment (Figure 1A). By contrast, leaves from AtMEK5KR, AtMEK5WT and Vector transgenic plants after DEX treatment, and leaves from AtMEK5DD AtMEK5KR, AtMEK5WT and Vector transgenic plants that had been incubated in distilled water with 0.05% ethanol (solvent of DEX), showed no sign of cell death (Figure 1A). We also used electrolyte leakage, an indicator of membrane damage, to show the extent of cell death. As shown in Figure 1B, electrolyte started to leak from leaves from AtMEK5DD transgenic plants at 24 h after DEX treatment. This result also demonstrates that cell death starts in leaves from AtMEK5DD plants at 24 h after DEX treatment. The ethylene biosynthesis was measured at various time points, as indicated. There was a dramatic increase in ethylene biosynthesis in leaves from AtMEK5DD plants (Figure 1C). Ethylene could be detected in leaves 4 h after DEX treatment. Thereafter, the rate of ethylene production progressively increased and reached its highest level after 20 h. By contrast, over the entire period, ethylene was not detected in leaves of any control plants (Figure 1C). Using an in-gel kinase assay, it can be seen that the expression of AtMEK5DD leads to rapid activation of endogenous AtMAPK3 and AtMAPK6, whereas expression of AtMEK5KR and AtMEK5WT only shows a basal level of AtMAPK6 activity (Figure 1D, left). Immunoblot analysis with anti-Flag antibody revealed that the expression levels of AtMEK5DD, AtMEK5WT and AtMEK5KR induced by DEX are similar (Figure 1D, right). These data indicate that ethylene biosynthesis can be induced by activation of AtMEK5 and the downstream MAPKs AtMAPK3 and AtMAPK6. Furthermore, ethylene biosynthesis occurs earlier than HR-like cell death.
Induction of AtMEK5 expression in transgenic Arabidopsis plants leads to endogenous AtMAPK3 and AtMAPK6 activation, ethylene biosynthesis, electrolyte leakage and cell death. (A) The leaves from AtMEK5DD, AtMEK5KR, AtMEK5WT and Vector control transgenic plants were stained with Evans blue after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. Cell death is indicated in blue. (B) Electrolyte leakage is associated with cell death in transgenic plants. Measurements of electrolyte leakage were performed as described in the Materials and Methods section. The results present the means and standard deviations from triplicate samples. (C) A time course analysis of ethylene production in leaves from transgenic Arabidopsis plants treated with DEX. The results present the means and standard deviations from triplicate samples. (D) A time course analysis of AtMEK5 mutants induction and endogenous AtMAPK6 and AtMAPK3 activation in leaves from transgenic Arabidopsis plants after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. The expression of AtMEK5 mutants was monitored by immunoblot analysis using anti-Flag M2 antibody (right). The kinase activity of endogenous AtMAPK3 and AtMAPK6 was determined by an in-gel kinase assay (left).
Kinetics of transcript accumulation for genes of ethylene biosynthesis and the ethylene responses that are induced by the activation of AtMEK5
Stress-stimulated ethylene production occurs owing to an increase in the activity of ACOs and ACSs, which is caused by post-translational regulation or transcriptional activation 18, 35, 36. The key steps of ethylene biosynthesis are controlled by ACS and ACO 28, 29. The basal activity of ACS is very low in tissues that do not produce significant amounts of ethylene, and ACO activity is constitutively present in most vegetative tissues. Therefore, the accelerated ethylene biosynthesis that occurs after stimulation is thought to be associated with an increase in the activity of ACS, which is caused by post-transcriptional regulation or the activation of ACS and ACO gene expression 28, 29, 34, 37. In order to determine whether there is a correlation between AtMEK5-induced ethylene production and the transcription of ACS and ACO, their transcript levels were monitored by Q-PCR. We probed for the eight genes that encode activated ACS protein in Arabidopsis 37, 38, 39, 40. However, the transcript levels of ACS2, ACS4, ACS7, ACS9 and ACS11 could not be detected in leaves from AtMEK5DD, AtMEK5KR, AtMEK5WT and Vector transgenic plants after DEX treatment. These results are in agreement with those reported previously 39. The transcript levels of ACS5 and ACS8 cannot be monitored by Q-PCR because of multiple bands of amplification when using RT-PCR. Therefore, we can only report the transcript level for ACS6 from Q-PCR. The transcript level of ACS6 was induced strongly in AtMEK5DD plants after DEX treatment (Figure 2). The transcript level of ACS6 following DEX treatment began to increase after 8 h and reached its peak after 20 h. The overall increase in transcript level was fifteen-fold. We also monitored the transcript levels of ACO1 and ACO2, which are two functionally characterized genes of the ACO gene family in Arabidopsis 41, 42. ACO1 was upregulated, while ACO2 was downregulated in AtMEK5DD plants (Figure 2). The transcript level of ACO1 following DEX treatment began to increase after 8 h and increased eight-fold to reach its peak after 12 h. These results suggest that induced transcription of ACS6 and ACO1 may also contribute to the increased ethylene production that is caused by the activation of AtMEK5.
Induction of ACS and ACO gene transcription in AtMEK5DD, AtMEK5KR, AtMEK5WT and Vector control transgenic plants after DEX treatment. The samples from transgenic plants were collected at the indicated times and then frozen in liquid nitrogen. RNA extraction and Q-PCR were performed as described in the Materials and Methods section. The results represent the means and standard deviations from triplicate samples.
To determine the response to elevated ethylene production that is induced by AtMEK5, we assessed the expression of ERF1, GST2 and CHIb, which have been reported to be downstream responsive genes of the ethylene pathway 43, 44. The transcript levels of the three genes were induced markedly by DEX treatment in AtMEK5DD plants (Figure 3). The transcript levels of the three genes peaked at 20 h after DEX treatment. These data indicate that the ethylene production that is induced by the activation of AtMEK5 is sufficient to induce further downstream responses to ethylene.
Induction of ethylene response gene transcription in AtMEK5DD, AtMEK5KR, AtMEK5WT and Vector control transgenic plants after DEX treatment. The samples from transgenic plants were taken at the indicated times and frozen in liquid nitrogen. RNA extraction and Q-PCR were performed as described in the Materials and Methods section. The results represent the means and standard deviations from triplicate samples.
Inhibition of ethylene biosynthesis and perception effectively delays the cell death that is induced by the activation of AtMEK5
To examine whether an increase of ethylene production has a role in subsequent cell death, cell death and ethylene production were measured in the presence of DEX, inhibitors of ethylene biosynthesis and an ethylene perception antagonist. Ethylene production was reduced considerably in AtMEK5DD transgenic plants treated with DEX after the addition of aminoethoxyvinylglycine (AVG), an inhibitor of ACSs, and CoCl2, an inhibitor of ACOs (Figure 4C). The level of ethylene production in AtMEK5DD transgenic plants treated with DEX and silver thiosulfate (STS), which is an ethylene perception antagonist, was similar to the level in plants treated with DEX only (Figure 4C). In the presence of AVG, CoCl2 and STS, the extent of cell death was reduced efficiently at 36 h and 48 h, while the whole leaf was dead at 72 h after DEX treatment. Cell death did not occur after treatment with AVG, CoCl2 and STS in the absence of DEX (Figure 4A). As shown in Figure 4B, the extent of electrolyte leakage from leaves of AtMEK5DD transgenic plants treated with inhibitors plus DEX was much lower than in AtMEK5DD transgenic plants treated with DEX only. This result also demonstrated that the extent of cell death was reduced by inhibitors of ethylene biosynthesis and perception. The inhibitors did not affect either the expression of AtMEK5DD, as determined by immunoblot analysis with an anti-Flag antibody (Figure 4D, right), or the activity of the downstream MAPKs (AtMAPK3 and AtMAPK6), as measured using an in-gel kinase assay (Figure 4D, left). These results suggest that activation of ACS and ACO activity is required for AtMEK5-induced ethylene production. In addition, ethylene production and perception may be required for AtMEK5-induced cell death.
Inhibition of ethylene biosynthesis and perception delayed the onset of AtMEK5-induced cell death. (A) Cell death induced by AtMEK5DD expression was delayed by inhibitors of ethylene biosynthesis and perception. Leaves from AtMEK5DD transgenic plants were pretreated with 2 μM AVG, 100 μM CoCl2 and 10 μM STS as described in the Materials and Methods section. Cell death was monitored by staining the leaves with Evans blue at 36, 48 and 72 h after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. (B) Electrolyte leakage is associated with cell death in AtMEK5DD transgenic plants. The results present the means and standard deviations from triplicate samples. (C) Ethylene production induced by AtMEK5DD expression was inhibited significantly by the ethylene biosynthesis inhibitors AVG and CoCl2, but not by the ethylene perception inhibitor STS. The results represent the means and standard deviations from triplicate samples. (D) A time course analysis of AtMEK5DD induction and endogenous AtMAPK6 and AtMAPK3 activation in leaves from AtMEK5DD transgenic Arabidopsis plants in the presence of AVG, CoCl2 and STS after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. The expression of AtMEK5DD was monitored by immunoblot analysis using anti-Flag M2 antibody (right). The kinase activity of endogenous AtMAPK3 and AtMAPK6 was determined by an in-gel kinase assay (left).
Ethylene insensitivity delays the cell death that is induced by the activation of AtMEK5
To demonstrate that ethylene perception and signaling are required for cell death, we introduced AtMEK5DD into the ethylene-insensitive mutants etr1-1 and ein2-1. The successful crosses were verified using the triple response (Figure 5A), hygromycin resistance and western blot methods (data not shown). Cell death in the leaves of AtMEK5DD/etr1-1 and AtMEK5DD/ein2-1 plants was reduced markedly in comparison to the leaves of AtMEK5DD plants at 36 h and 48 h, while the whole leaf was dead at 72 h after DEX treatment (Figure 5B). As shown in Figure 5C, the extent of electrolyte leakage from the leaves of AtMEK5DD/etr1-1 and AtMEK5DD/ein2-1 plants was much lower than from those of AtMEK5DD plants after DEX treatment. This result also demonstrates that the extent of cell death was reduced in ethylene insensitive mutants. Ethylene production was reduced slightly in AtMEK5DD/etr1-1 and AtMEK5DD/ein2-1 plants (Figure 5D), which may be due to feedback regulation of ethylene biosynthesis by the ethylene response. The comparable AtMEK5DD protein and activities of downstream MAPKs were shown in AtMEK5DD/etr1-1, AtMEK5DD/ein2-1 and AtMEK5DD plants (Figure 5E). These data confirmed that ethylene signaling perception is required for the regulation of AtMEK5-induced cell death.
Ethylene insensitivity delayed the onset of AtMEK5-induced cell death. (A) A triple response of AtMEK5DD, AtMEK5DD/etr1 and AtMEK5DD/ein2 plants in the presence or absence of 1 mM ACC. (B) Cell death induced by the activation of AtMEK5 was delayed in AtMEK5DD/etr1 and AtMEK5DD/ein2 plants. Cell death was monitored by staining the leaves from AtMEK5DD, AtMEK5DD/etr1 and AtMEK5DD/ein2 plants with Evans blue at 36, 48 and 72 h after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. (C) Electrolyte leakage is associated with cell death in AtMEK5DD/etr1 and AtMEK5DD/ein2 transgenic plants. The results present the means and standard deviations from triplicate samples. (D) Ethylene production in AtMEK5DD/etr1 and AtMEK5DD/ein2 after DEX (as +DEX) or 0.05% ethanol (as −DEX) treatments. The rates of ethylene production were measured at the times indicated. Results represent the means and standard deviations of triplicate samples. (E) A time course analysis of AtMEK5DD induction and endogenous AtMAPK6 and AtMAPK3 activation in leaves from AtMEK5DD, AtMEK5DD/etr1 and AtMEK5DD/ein2 plants. The expression of AtMEK5DD was monitored by immunoblot analysis using anti-Flag M2 antibody (right). The kinase activity of endogenous AtMAPK3 and AtMAPK6 was determined by an in-gel kinase assay (left).
Discussion
A diverse array of stresses, which include pathogen infection, wounding, UV irradiation, drought, high osmolarity, ozone and oxidative stress, have been shown to activate AtMAPK3 or AtMAPK6, or both, in Arabidopsis and their orthologs in other plant species 12, 26, 45, 46. These stresses have also been reported to induce ethylene production 18, 28, 34, 47, 48, 49, 50. The long-lasting activation of AtMAPK6 alone or both AtMAPK3 and AtMAPK6 by their upstream MAPKKs (namely AtMEK4 and AtMEK5 in Arabidopsis, and their ortholog NtMEK2 in tobacco) induces HR-like cell death 20, 24. Expression of the NtMEK2 active mutant also induces an impressive increase in ethylene production in transgenic tobacco and Arabidopsis 18, 20, 24, 34. This ethylene production is due to the increased activity of ACS. The increased activity of ACS is caused by the accumulation of phosphorylated ACS2/ACS6 34 and newly translated ACS2/ACS6 18. However, the role of ethylene in cell death has not yet been elucidated. The results of this investigation have established that the expression of AtMEK5DD, which is an active mutant of AtMEK5, induces a striking increase in ethylene production (Figure 1C). We have also explored the role of ethylene in the HR-like cell death that is caused by AtMEK5.
Various lines of evidence show that ethylene biosynthesis and/or ethylene signaling are involved in the regulation of cell death. Studies involving the use of inhibitors of ethylene biosynthesis 51, 52, 53 ethylene perception antagonists 47, 53, 54 and ethylene-insensitive mutants with deficiencies in ethylene signaling 54 have revealed significantly reduced lesion formation and expansion in leaves that are exposed to ozone. The role of ethylene in the pathogenesis of disease and defense responses in infected plants has been studied extensively 28, 55. For example, the onset of HR-like cell death in tobacco infected with TMV 18, 56, tomato infected with Cladosporium fulvum 57 and rice infected with Magnaporthe grisea 58 was accompanied by a large burst of ethylene production 59. Significant suppressed TMV-induced HR-like cell death and expansion was observed in tobacco treated with inhibitors of ethylene biosynthesis or ethylene perception antagonists 56, 60. Knoester and colleagues reported that ethylene-insensitive tobacco (Tetr) plants, transformed with a mutant etr1-1 gene from Arabidopsis, had reduced virus-induced lesion expansion 56. We observed that HR-like cell death began at the leaf edges of AtMEK5DD plants at 24 h, and that the whole leaf was dead at 36 h after DEX treatment (Figure 1A). This start time of cell death is the same as has been reported previously 24. However, the time at which an increase in ethylene could be detected was well before the start of cell death (Figure 1). Kim et al. reported that the expression of NtMEK2DD, which is an active mutant of NtMEK2, induced the ethylene biosynthesis before the appearance of HR-like cell death in tobacco 18. These results suggest that AtMEK5- and NtMEK2-induced ethylene production is not a consequence of cell death.
To appreciate the role of ethylene in AtMEK5-induced cell death, we performed two sets of experiments. First, we monitored the onset of cell death using CoCl2 and AVG, two widely used inhibitors of ethylene biosynthesis. If the cell death was ethylene-independent, we would expect that the time of whole-leaf death would not be affected after inhibiting ethylene biosynthesis. However, we observed that cell death was delayed considerably when ethylene biosynthesis was blocked by two inhibitors (Figure 4A). Second, we blocked ethylene signaling by using the ethylene receptor antagonist STS and the ethylene insensitive mutants etr1 and ein2. The time of whole-leaf death was delayed in the presence of STS and when AtMEK5DD was introduced into ethylene insensitive mutants etr1 and ein2 (Figure 4A and Figure 5B). On the basis of our results, we concluded that ethylene signaling is required for the acceleration of AtMEK5-induced cell death.
Inhibiting ethylene biosynthesis or blocking ethylene signaling significantly delayed, but did not completely abolish cell death, which suggests that other signaling pathway(s) might also be involved in the regulation of AtMEK5-induced cell death. H2O2 has been established as an important trigger for HR-like cell death in plants that are challenged with pathogen 61. The accumulation of H2O2 has been shown to be required for the cell death that is caused by other stresses 53. Since large amounts of H2O2 are generated in AtMEK5-induced cell death 24, H2O2 may be an important component in causing cell death. Therefore, we tried to explore the role of H2O2 in cell death by using inhibitors, such as diphenyleneiodonium, KCN and NaN3, to block the production of H2O2. Unfortunately, we were unable to reach any conclusions from these experiments (data not shown) because the inhibitors themselves also caused cell death in controls. To further elucidate the role of H2O2 in cell death, AtMEK5DD needed to be transformed and expressed in mutants that are impaired in H2O2 generation or defective in H2O2 signaling.
Materials and Methods
Plant growth conditions and treatments
Wild-type and transgenic Arabidopsis thaliana (Columbia ecotype) plants were grown in a growth chamber at 22 °C with a 12 h light : 12 h dark photoperiod. Fully expanded leaves of 4-week-old plants were used for the experiments.
Transgene expression was induced by incubating the leaves in 15 μM of DEX (Sigma) solution. For treatment with inhibitors of ethylene biosynthesis or receptors, the leaves were incubated for 2 h in 100 μM of CoCl2, or 2 μM of AVG (Sigma), or 10 μM of STS (freshly prepared before use from stock solutions of STS and silver nitrate in a 4:1 molar ratio) before DEX was added. Samples were harvested at the indicated time. The samples were frozen in liquid nitrogen and stored at −80 °C until use.
Measurement of ethylene content in leaves
Detached leaves (∼0.3 g) were treated with DEX in a 10 ml vial sealed with a rubber plug. 1 ml of the gas was removed from the vial and introduced to a gas chromatograph equipped with a flame ionization detector (GC-7AG, Shimadzu, Tokyo, Japan). The amount of ethylene was estimated by comparing it with an ethylene standard. The data are displayed as the mean ± the standard deviation from triplicate samples. All the experiments were repeated for three times.
Generation of crosses
Arabidopsis mutants of etr1-1 (CS237, on a Col-0 background) and ein2-1 (CS3071, on a Col-0 background) were obtained from ABRC. The etr1-1 and ein2-1 mutants were crossed into an AtMEK5DD transgenic background. AtMEK5DD was identified using hygromysin resistance and transgene expression. The etr1-1 and ein2-1 mutants were identified on the basis of the ethylene-insensitive phenotype. Homozygous F3 plants were used for the experiments.
Detection of cell death in leaves using Evans blue staining
Cell death was measured in the leaves by staining with Evans blue according to the method described by Baker and Norton 62, but with some modifications. Leaves were taken at the indicated times and soaked in 10 ml of 0.25% Evans blue. They were then washed briefly in 10 ml water. Leaves were de-stained in boiling 96% ethanol for 10 min. Then the leaves were transferred to a 60% glycerol solution.
Measurement of electrolyte leakage from leaves
At various times after treatment about 0.1 g of leaves was sampled from the plants. After brief rising, the leaves were shaken in 3 ml of distilled water on a rotary shaker at 100 rpm for 1 h at room temperature. The conductivity of the solution was measured with a conductivity meter (model 3173R, JENCO, China).
Quantitative RT-PCR analysis
Total RNA was extracted from samples using Trizol (Invitrogen) according to the manufacturer's instructions. Reverse transcription (RT) was performed using 2.4 μg of total RNA and 0.6 μg of oligo (dT)16 primer with M-MLV reverse transcriptase (Promega). Aliquots of the products of RT were used as a template for quantitative PCR (Q-PCR). Q-PCR was run with SYBR green fluorophore (Molecular Probes), 100 nM primer and a 1 μl aliquot of RT product in a 20 μl final volume per reaction. PCR analysis was performed using a DNA Engine Opticon II system (MJ Research) according to the manufacturer's instructions. Agarose gel electrophoresis and thermal denaturation (melt curve analysis) were used to confirm specific replicon formations. For each reaction, the threshold cycle value (CT) was determined by setting the threshold within the logarithmic amplification phase. The CT values were converted to relative gene expression levels using the 2-ΔCT method. After normalization to an Actin8 control, the relative levels of gene expression were calculated. Q-PCR reactions were done in triplicate and averaged for each time point. Primer pairs used for Q-PCR were:
ACS6 (At4g11280) forward: 5′-CCG GGA ATG TTT GAA GTC TCT TG-3′;
ACS6 reverse: 5′-CGG TCT TAA GTC TGT GCA CGG-3′;
ACO1 (At1g05010) forward: 5′-TCC CGA TCA TCA ATC TCG AG-3′;
ACO1 reverse: 5′-CAG TGG CCA ATG GTC CAA C-3′;
ACO2 (At1g62380) forward: 5′-CAG TGG CCA ATG GTC CAA C-3′
ACO2 reverse: 5′-CTA ACC TAT ACG ACA TCC CTG-3′
ERF1 (At3g23240) forward: 5′-CGG CGG AGA GAG TTC AAG AGT C-3′;
ERF1 reverse: 5′-TCC CAC TAT TTT CAG AAG ACC CC-3′;
GST2 (At4g02520) forward: 5′-ATG GCA GGT ATC AAA GTT TTC GG-3′;
GST2 reverse: 5′-ATT TCT CAC TGA ACC TTC TCG-3′;
CHI-b (At3g12500) forward: 5′-ATC ACC GCT GCA AAG TCC TTC-3′;
CHI-b reverse: 5′-TGC TGT AGC CCA TCC ACC TG-3′;
Actin 8 (At1g49240) forward: 5′-GGT GAT GGT GTG TCT-3′;
Actin 8 reverse: 5′-ACT GAG CAC AAT GTT AC-3′.
Protein extraction and immunoblot analysis
Total protein was extracted from leaf tissues by grinding with plastic pestles in extraction buffer (100 mM HEPES, pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerophosphate, 10 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin and 5% glycerol). After centrifugation at 10 000 g for 20 min, supernatants were transferred into clean tubes. The protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad), using bovine serum albumin as the standard. For western blot analysis, 7.5 μg of total protein per lane was separated on a 10% SDS polyacrylamide gel (SDS-PAGE). After electrophoresis, the proteins were electro-transferred onto nitrocellulose membranes. After blocking for 1 h in TBST buffer (50 mM Tris-HCl, pH7.5, 150 mM NaCl, 0.05% Tween 20) with 5% fat-free dried milk at room temperature, the membranes were incubated with anti-Flag antibody M2 (Sigma, 1:10 000 dilution) for 2 h. Following three washes with TBST buffer, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG as secondary antibody (Sigma, 1:10 000 dilution). After three washes with TBST buffer, the membranes were then visualized using an enhanced Lumi-Light Western Blotting Substrate kit (Roche), following the manufacturer's instructions.
In-gel kinase activity assay
The in-gel kinase activity assay was performed using a previously described method 24. In brief, an aliquot containing 7.5 μg of protein extract was electrophoresed on 10% SDS-polyacrylamide gels embedded with 0.1 mg/ml myelin basic protein in separating gel as a substrate for kinase. After electrophoresis, the SDS was removed from the gel by washing with washing buffer (25 mM Tris-HCl, pH 7.5, 0.5 mM dithiothreitol, 0.1 mM Na3VO4, 5 mM NaF, 0.5 mg/ml bovine serum albumin, and 0.1% Triton X-100) three times for 30 min each at room temperature. The proteins were then renatured in 25 mM Tris-HCl, pH 7.5, 1 mM dithiothreitol, 0.1 mM Na3VO4, 5 mM NaF at 4 °C overnight with three changes of the buffer. The gel was incubated at room temperature in 100 ml of reaction buffer (25 mM Tris-HCl, pH 7.5, 2 mM EGTA, 12 mM MgCl2, 1 mM dithiothreitol, 0.1 mM Na3VO4) for 30 min. Phosphorylation was performed for 1.5 h at room temperature in 30 ml reaction buffer that contained 200 nM ATP and 50 μCi of γ-32P-ATP (3 000 μCi/mmol). The reaction was stopped by transferring the gel into a solution of 5% trichloroacetic acid (w/v) and 1% sodium pyrophosphate (w/v). The unincorporated radioactivity was subsequently removed by washing the gel for 5 h at room temperature with five changes. The gel was dried on Whatman 3MM paper and subjected to autoradiography. Pre-stained size markers (NEB) were used to calculate the sizes of the kinases.
References
Pennell RI, Lamb C . Programmed cell death in plants. Plant Cell 1997; 9:1157–1168.
Overmyer K, Brosche M, Kangasjarvi J . Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 2003; 8:335–342.
Dangl JL, Jones JDG . Plant pathogens and integrated defence responses to infection. Nature 2001; 411:826–833.
Dixon RA . Natural products and plant disease resistance. Nature 2001; 411:843–847.
Glazebrook J . Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 2005; 43:205–227.
Greenberg JT, Yao N . Microreview The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 2004; 6:201–211.
Martin GB, Bogdanove AI, Sessa G . Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 2003; 54:23–61.
Torres MAD, Jeffery L . Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 2005; 8:397–403.
Zhang S, Klessig DF . Pathogen-induced MAP kinase in tobacco. Res Prob Cell Differ 2000; 27:65–84.
Somssich IE, Hahlbrock K . Pathogen defence in plants — a paradigm of biological complexity. Trends Plant Sci 1998; 3:86–90.
Scheel D . Resistance response physiology and signal transduction. Curr Opin Plant Biol 1998; 1:305–310.
Jonak C, Okresz L, Bogre L, Hirt H . Complexity, Cross talk and integration of plant MAP Kinase signalling. Curr Opin Plant Biol 2002; 5:415–424.
Chang L, Karin M . Mammalian MAP kinase signalling cascades. Nature 2001; 410:37–40.
Widmann C, Gibson S, Jarpe MB, Johnson GL . Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999; 79:143–180.
Nakagami H, Pitzschke A, Hirt H . Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 2005; 10:339–346.
Pedley KF, Martin GB . Role of mitogen-activated protein kinases in plant immunity. Cur Opin Plant Biol 2005; 8:541–547.
Liu Y, Jin H, Yang KY, et al. Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J 2003; 34:149–160.
Kim CY, Liu Y, Thorne ET, et al. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 2003; 15:2707–2718.
Hirt H . MAP kinase in plant signal transduction. Res Prob Cell Differ 2000; 27:1–9.
Yang KY, Liu Y, Zhang S . Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 2001; 98:741–746.
Ren D, Yang KY, Li GJ, Liu Y, Zhang S . Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol 2006; 141:1482–1493.
Zhang S, Liu Y . Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 2001; 13:1877–1889.
Jin H, Liu Y, Yang KY, et al. Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J 2003; 33:719–731.
Ren D, Yang H, Zhang S . Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 2002; 277:559–565.
Pedley KF, Martin GB . Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J Biol Chem 2004; 279:49229–49235.
MAPK group. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 2002; 7:301–308.
Arshad M, Frakenberger WT . Ethylene. Dordrecht, NL: Kluwer Academic Press 2002:11–50.
Wang KLC, Li H, Ecker JR . Ethylene biosynthesis and signaling networks. Plant Cell 2002; 14:S131–S151.
Kende H . Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 1993; 44:283–307.
Adams DO, Yang SF . Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 1979; 76:170–174.
Spanu P, Grosskopf DG, Felix G, Boller T . The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol 1994; 106:529–535.
De Laat AMM, Van Loon LC . The relationship between simulated ethylen production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol 1983; 22:261–273.
Zhang S, Klessig DF . Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc Natl Acad Sci USA 1998; 95:7433–7438.
Liu Y, Zhang S . Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 2004; 16:3386–3399.
Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux JP, Broekaert WF . Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998; 10:2103–2114.
Cohn JR, Martin GB . Pseudomonas syringae pv. tomato type III effectors AvrPto and AvrPtoB promote ethylene-dependent cell death in tomato. Plant J 2005; 44:139–154.
Chae HS, Kieber JJ . Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 2005; 10:291–296.
Liang X, Abel S, Keller JA, Shen NF, Theologis A . The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana. Proc Natl Acad Sci USA 1992; 89:11046–11050.
Yamagami T, Tsuchisaka A, Yamada K, et al. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 2003; 278:49102–49112.
Tsuchisaka A, Theologis A . Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc Natl Acad Sci USA 2004; 101:2275–2280.
Raz V, Ecker JR . Regulation of differential growth in the apical hook of Arabidopsis. Development 1999; 126:3661–3668.
Gomez-Lim MA, Valdes-Lopez V, Cruz-Hernandez A, Saucedo-Arias LJ . Isolation and characterization of a gene involved in ethylene biosynthesis from Arabidopsis thaliana. Gene 1993; 134:217–221.
Ouaked F, Rozhon W, Lecourieux D, Hirt H . A MAPK pathway mediates ethylene signaling in plants. EMBO J 2003; 22:1282–1288.
Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR . EIN2, a Bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 1999; 284:2148–2152.
Zhang S, Klessig DF . MAPK cascades in plant defense signaling. Trends Plant Sci 2001; 6:520–527.
Tena G, Asai T, Chiu WL, Sheen J . Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 2001; 4:392–400.
Vahala J, Ruonala R, Keinanen M, Tuominen H, Kangasjarvi J . Ethylene insensitivity modulates ozone-induced cell death in birch. Plant Physiol 2003; 132:185–195.
Mckeon TA, Fernandez-Maculet LC, Yang SF . Biosynthesis and metabolism of ethylene. In Plant hormones. PJ Davies ed. Dordrecht, NL: Kluwer Academic Press 1995:118–139.
Johnson PR, Ecker JR . The ethylene gas signal transduction pathway: A molecular perspective. Annu Rev Genet 1998; 32:227–254.
Bleecker AB, Kende H . Ethylene: A gaseous signal molecule in plants. Annu Rev Cell Dev Biol 2000; 16:1–18.
Wenzel AA, Schlautmann H, Jones CA, Kuppers K, Mehlhorn H . Aminoethoxyvinylglycine, cobalt and ascorbic acid all reduce ozone toxicity in mung beans by inhibition of ethylene biosynthesis. Physiol Plant 1995; 93:286–290.
Tuomainen J, Betz C, Kangasjarvi J, et al. Ozone induction of ethylene emission in tomato plants: regulation by differential accumulation of transcripts for the biosynthetic enzymes. Plant J 1997; 12:1151–1162.
Moeder W, Barry CS, Tauriainen AA, et al. Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 2002; 130:1918–1926.
Overmyer K, Tuominen H, Kettunen R, et al. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 2000; 12:1849–1862.
Dong X . SA, JA, ethylene, and disease resistance in plants. Cur Opin Plant Biol 1998; 1:316–323.
Knoester M, Linthorst HJM, Bol JF, Van Loon LC . Involvement of ethylene in lesion development and systemic acquired resistance in tobacco during the hypersensitive reaction to tobacco mosaic virus. Physiol Mol Plant Pathol 2001; 59:45–57.
Hammond-Kosack KE, Jones JDG . Resistance gene-dependent plant defense responses. Plant Cell 1996; 8:1773–1791.
Iwai T, Miyasaka A, Seo S, Ohashi Y . Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 2006; 142:1202–1215.
Van Loon LC, Geraats BPJ, Linthorst HJM . Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 2006; 11:184–191.
Ohtsubo N, Mitsuhara I, Koga M, Seo S, Ohashi Y . Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol 1999; 40:808–817.
Levine A, Tenhaken R, Dixon R, Lamb C . H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994; 79:583–593.
Baker CJ, Norton MM . An improved method for monitoring cell death in cell suspension and leaf disc assays using evans blue. Plant Cell Tiss Org 1994; 39:7–12.
Acknowledgements
This work was supported by National Basic Research Program of China (Grant No. 2003CB114304), the National Natural Science Foundation of China (Grant Nos. 30421002, 30770203, and 30370140), NCET 04-0131 and the Fok Ying Tung Education Foundation (Grant No. 91022) to D Ren, and the National Natural Science Foundation of China (Grant No. 30770128) to G Liu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Wang, Y., Xu, J. et al. Ethylene signaling is required for the acceleration of cell death induced by the activation of AtMEK5 in Arabidopsis. Cell Res 18, 422–432 (2008). https://doi.org/10.1038/cr.2008.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2008.29