Abstract
This paper probes into the feasibility of increasing expression level of hFIX gene with endogenous intron 1 sequence, hFIX minigene was obtained with middle sequence truncated intron 1 inserted into the relative site of hFIX cDNA, and plasmid vector pKG5i'IX, retroviral vector GINaCi'IX were constructed. These vectors were transduced into target cells of PA317, C2C12, primary rabbit skin fibroblasts (RSF) and primary human skin fibroblasts (HSF). The expression level of mixed colonies are PA317/pKG5i'IX, 151 ng/106 cells/24h; PA317/G1NaCi'IX, 308 ng/106 cells/24 h; C2C12/G1 NaCi'IX, 186 ng/106 cells/24 h; RSF/G1NaCi'IX, 1929 ng/106 cells/24 h; HSF/G1NaCi'IX, 1646 ng/106 cells/24 h. These results indicated that hFIX minigene with intron 1 is able to increase the expression level to about 3 times of that of hFIX cDNA. Meanwhile, in order to study the application of hFIX minigene in the retroviral-mediated gene transfer system and refrain from intron splicing during viral production, a retroviral vector G1NaCi'IXR with reversely inserted hFIX minigene expression cassette was constructed. The expression level of reverse constructor in PA317 cells was 390 ng/106 cells/24 h with 79 % of bioactivity. PCR detection of HT/G1NaCi'IXR cells infected with PA317/G1NaCi'IXR supernatant confirmed the existence of intron 1 sequence. These results suggested that expression vector with forward-inserted intronl-carrying hFIX expression cassette can be used in directed gene transfer, but when using the retroviral-mediated gene transfer system, reversely-inserted intronl-carrying hFIX expression cassette should be considered.
Similar content being viewed by others
Introduction
Hemophilia B, a serious bleeding disorder, is an inherited X-chromosome linked disease caused by the deficiency or inactivity of the clotting factor IX (hFIX). The incidence of this disease is about one in 100,000 males. With the conventional transfusion of blood, thrombinogen complex or concentrated factor IX, the patients' symptoms could be relieved to some extent, but these treatments are expensive and may cause serious reactions and increase the risks of infection with HIV or hepatitis virus1.
Since 1987, Hemophilia B became a potential candidate for gene therapy, and promising achievements had been made2, 3, 4, 5, 6, 7, 8. From 1991, the Stage I Clinical Trial of Hemophilia B for Gene Therapy had been successfully carried out by our lab on two hemophilia B patients9, 10, 11, 12, 13. After injection of autologous skin fibroblasts transduced with expression vector with human clotting factor IX eDNA , the symptoms of patients relieved with the concentration and bioactivity of factor IX increased from 71 ng/ml and 2.9 % to 240 ng/ml and 6.3 %14 respectively.
It must be mentioned that in our previous trial, the concentration of hFIX could only reach to 5% of normal value (5000 ng/ml), and each treatment maintained for 1–1.5 years with the symptoms of the patients alleviated from middle grade to minor grade hemophiliac. So, in order to cure hemophilia B patient , that is, to increase the concentration of hFIX of patients to 10% of normal value to have the therapeutic effect, it is necessary to study the regulation of hFIX expression further. A number of specific regulation factors existed in the intron sequence of eukaryotic genes were studied15, and intron 1 sequence of hFIX was required in the expression of hFIX in transgenic mice16, hFIX cDNA had been used in our previous studies, now we expect to increase the expression level by inserting an intron signal sequence into expression vector to stimulate the normal psychological condition of hFIX, which may prolong the maintaining time, and deliminate the deference of expression between in vivo and in vitro.
In this study, a series of hFIX minigene which carrying endogenous truncated intron 1 sequence were constructed and transduced into different target cells, the expression level of those cells were compared with that of cells transduced with hFIX cDNA. Besides, the phenomenon of intron splicing during virus production of retroviral mediated gene transfer system was also studied.
Materials and Methods
Materials
-
1
Various restriction enzymes and DNA ligase were purchased from New England Biolabs; proteinase K and CIP were purchased from Boehringer-Mannheim; FD DNA polymerase was produced by Science and Technology Advancement Company of Fudan University; RT-PCR Kits was purchased from Promega company.
-
2
Plasmids G 1NaCIX was constructed in our lab17; pKG5IX and pBSIX were kindly provided by Prof. Brownlee of Oxford University2; plPSTI was provided by Dr. Jin J.P. of University of North Carolina18.
-
3
PA317 was purchased from ATCC18; human primary skin fibroblasts were cultured in our lab; cell culture medium DMEM and a MEM were purchased from GIBCO.
-
4
Mouse anti-human FIX monoantibody 3A6 was provided by Dr. Yoshioka of Nara Medical College in Japan19. Rabbit anti-human FIX antiserum and peroxidase-conjugated sheep antirabbit IgG antiserum were purchased from Cabbiochem-Berhing.
-
5
PCR primers were synthesized by Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
Methods
-
1
Recombinant vector construction, plasmid DNA extraction, Digestion, electrophoresis, DNA fragment recovery, ligation and transformation, and etc. were carried out as routine methods.
-
2
Cell culture: PA317, C2C12 and HT cells were cultured in DMEM medium with 10 % fetal bovine serum, 100 μg/ml penicillin and 100 μg/ml streptomycin, and incubated in 5% CO2; human primary skin fibroblasts were cultured in α MEM with 20 % fetal bovine serum.
-
3
Calcium phosphate transformation, cell colony selection and determination of hFIX protein in culture medium by ELISA: according to the method previously reported by Dai9.
-
4
RT-PCR were carried out based on the protocol supplied with Kits.
Results and Analysis
Construction of expression vector
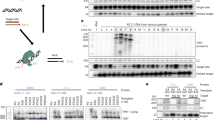
In order to study the effect of intron 1 on the expression of hFIX, a series of expression vectors, pKG5i'IX, G1NaCi'IX and G1NaCi'IXR were constructed. All those vectors included the hFIX intron 1 with the fragment from 701 to 6111 truncated. The 5′-end fragment of intron 1 was obtained from plasmid plPSTI and the 3′-end fragment was PCR amplified from one phage of the hFIX gene pool, FIX λ 61. pKG5i'IX was plasmid vector in which hFIX was driven by SV40 promoter, while other two vectors were retroviral vectors: G1NaCi'IX carried the forward inserted hFIX expression cassette while G1NaCi'IXR carried the reversely inserted hFIX expression cassette. In both retroviral vectors, hFIX expression sequence was driven by hCMV promoter. The construction of these three expression vectors are shown in figuer 1 (A: pKG5i'IX, B: G1NaCi'IX, C: G1NaCi'IXR).
Expression of hFIX in different cell types
Four vectors were transformed into retrovirus helper cell PA317, mouse myoblast C2C12, rabbit primary skin fibroblast RSF and human primary skin fibroblast HSF. Cells were selected by 300 μg/ml of G418 for two weeks, and the mixed colonies were trypsinized and implanted into plate with DMEM and 5 μg/ml of Vit. K1 at the number of 1 × 106. 24 h later, the expression level and bioactivity were detected by ELISA and Barium Citrate Absorption from the culture media Tab 1.
The results showed that hFIX minigene with intron 1 can increase the expression level to about 3 times of that of hFIX cDNA, while the bioactivity remains unchanged.
Approach of hFIX intron 1 to retroviral mediated transfer
It is necessary to approach hFIX intron 1 in the retroviral mediated gene transfer system, since the retroviral system is widely used for gene transfer and is confirmed to transduce target gene into cell genome.
It is supposed that intron 1 sequence of G1NaCi'IX will be spliced during the virus production because retrovirus is an RNA virus and intron can be spliced in RNA level. It is reasonable to avoid splicing by reversely inserting the hFIX minigene sequence into the backbone of retroviral vector, thus during RNA synthesis, splice enzyme will be unable to recognize the splicing donor and acceptor site, and then intron will be remained and existed in the genome of target cell.
Such retroviral vector was constructed and named G1NaCi'IXR, when this vector was transformed into PA317 cells, the expression level of hFIX was 390 ng/106 cells/24 h with 79% of bio-activity.
To detect the existence of intron sequence, three primers were designed according to Fig 2. A 361 bp band can be amplified from the intron-containing sequence, while a 261 bp band can be amplified from the sequence containing cDNA sequence alone.
The cell culture and supernatant of PA317, PA317/G1NaCIX, PA317/G1NaCi'IX and PA317/G1NaCi'IXR were collected and detected by PCR and RT-PCR methods (Tab 2, Fig 3, 4). Results showed that intron spliced and unspliced sequence theoratically existed in the forwardly inserted hFIX minigene, but only the spliced sequence can be detected in the supernatant of PA317/G1NaCIX and PA317/G1NaCi'IX cells; intron unpliced sequence existed in the reversely inserted hFIX minigene, while intron sequence can be detected in the supernatant of PA317/G1NaCi'IXR cells.
HT cells were infected with the supernatant of PA317/G1NaCi'IX and PA317/G1-NaCi'IXR cells, G418 positive colonies were detected by PCR method. Results showed that a 261bp band was existed in HT/G1NaCi'IX cells and a 361 bp band was existed in HT/G1NaCi'IXR cells as expected. (Fig 5)
These results preliminarily confirmed that in retroviral mediated gene transfer system, an intron sequence can be detected in target cells only when the intron containing expression cassette was reversely inserted into the backbone of retroviral vector. Besides, forward inserted sequence is suggested to be used in direct gene transfer system.
Discussion
Effects of intron in gene expression
Intron regulates gene expression on both transcription and post-transcription level. On the transcription level, some regulation factors were found in intron sequence, mainly enhancers. Most of these sequences were DNase I hypersensitive site, and so were tissue specific. On the post-transcription level, intron splicing effects on the gene expression. During splicing, intron not only helps to format 5′-cap and 3′-polyA to increase the stability of mature mRNA, but protects the pre-RNA from the digestion of nuclear RNA enzyme, while binding to splisome20.
Based on the results of this paper and previous studies21, we suggested that the intron 1 of hFIX gene effects on the post-transcription level, because there is no enhancer element existed in intron 1, and truncated intron 1 can increase the expression level to three times in vitro.
Study of the mechanism of transforming retroviral vectors into PA317 cells with calcium phosphate method
During the process of cell transformation with calcium phosphate, we found that the efficiency of transforming retroviral vectors into package cells PA317 is higher than that of transforming plasmid into other cell lines. Besides, PCR detection in some of the G418 positive colonies of PA317/G1NaCi'IX showed that intron sequence has been deleted. Since it is a retroviral mediated gene transfer system which involves the transcription and may cause intron splicing, we concluded that these colonies were formed from retrovirus transduced cells. When retroviral vectors were transformed into PA317 cells, part of the viral particles were produced before G418 positive colonies formed. When these virus were secreted into supernatant, they are able to transduce other PA317 cells, thus the formed colonies may contain intron spliced hFIX sequence. Hence, there were two DNA transformation systems existed in the transformed PA317 with retroviral vectors by calcium phosphate method, and resulted in a high transfection efficiency.
Based on these observation, it is suggested to modify the routine protocal of retroviral gene transfer to eliminate the selection and amplification of colonies of virus-producing cells formed after transfection. Instead, the supernatant of PA317 cells 24-72 h after transfection would be used to transduce the target cells.
The construction of hFIX minigene
The minigene of hFIX we constructed in this paper is an initiated one, though it can increase the expression level to about three times of the hFIX cDNA. To construct a complete and efficiency minigene that can prolong a high level of expression in vivo, some factors must be considered.
An endogenous promoter or a promoter of mammalian house keeping gene,such as that of β actin gene is suggested.
5′-non coding sequence is considered to be added in the minigene, for + 1 – + 18 site is C/EBP binding site, and −26 — 20 is the tissue specific binding site of HNF422. Those two sites have the effect on the expression of hFIX in vivo.
Kozak sequence can be used by site mutation on existed sequence of hFIX to increase translation23.
To avoid the promoter locking phenomenon, IRES (ribosome re-entry site) sequence is suggested in the vector construction24.
References
Ragni MV, et al. 1986 update of HIV seroprevalence, serocoonversion, AIDS incidence, and immunologic correlates of HIV infection in patients with hemophilia A and hemophilia B. Blood 1987; 70:786–90.
Anson DS, Choo KH, Rees DJG, et al. The gene structure of human anti-hemophiliac factor IX. EMBO J 1984; 3(5):1053–60.
Yoshitake S, Schach BG, Foster DC, et al. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry, 1985; 24(14):3736–50.
Anson DS, Hock RA, Austen D, et al. Towards gene therapy for hemophilia B. Mol Biol Med 1987; 4:11–20.
St. Louis D . and Verma IM . An alternative approach to somatic cell gene therapy. PNAS 1988; 85:3150–4.
Palmer TD, Thompson AR, Miller AD . Production of human factor IX in animals by genetically modified skin fibroblasts: potential therapy for hemophilia B. Blood 1989; 73(2):438–45.
Scharfmann R, Axelrod JH, Verma IM, et al. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. PNAS 1991; 88:4626–30.
Liu HW, Ofosu FA, Chang PL, et al. Expression of human factor IX by microencapsulated recombinant fibroblasts. Hum Gene Ther 1993; 4:291–301.
Dai YF, Qiu XF, Xue JL, et al. High efficient transfer and expression of human clotting factor IX cDNA in cultured human primary skin fibroblasts form hemophilia B patient by retroviral vectors. Science in China (series B), 1993; 35(2):183–93.
Wang P, Dai YF, Qiu XF, et al. High expression of human factor IX cDNA dreven by hcmv promoter in mammalian cells. Chinese Science Bulletin 1992; 37(1):52–5.
Zhou JM, Hou GY, Hu YP, et al. Construction of recombinant plasmid containing human factor IX cDNA and its expression by transfected cells in mice. Chinese Science Bulletin 1993; 38(11):956–60.
Zhou JM, Dai YF, Qiu XF, et al. Expression of human factor IX cDNA in mice by implants of genetically modified skin fibroblasts from a hemophilia B patient. Science in China (series B) 1993; 36(9):1082–92.
Zh'ou JM, Qiu XF, Lu DR, et al. Long term expression of human factor IX cDNA in rabbits. Science in China (series B), 1993; 36(11):1333–41.
Lu DR, Zhou JM, Zheng B, et al. Stage I clinical trial of gne therapy for hemophilia B. Science in China (series B) 1993; 36(11):1342–51.
Jonsson JJ, Foresman MD, Wilson N, et al. Intron requirement for expression of the human purine nucleoside phosphorylase gene. Nucleic Acids Research, 1992; 20(12):3191–8.
Jallat S, Perraud F, Dalemans W, et al. Characterization of recombinant human factor IX expressed in transgenic mice and derived trans-immortallized hepatic cell lines. EMBO J 1990; 9:3295–301.
Lu DR . Study of hemophilia B gene therapy (Doctor's thesis). 1995
Ware J, Davis L, Frazier D, et al. Genetic defect responsible for the dysfunctional protein: factor IX Long Beach. Blood 1988; 72(2):820–2.
Yoshioka A . Immunoassays of factor IX antigenusing monoclonal antibodies. Bri J Haemat 1985; 59:265–75.
Qiu Xiaoyun, Lu Daru . Role of intron in the regulation of gene expression. Foreign Medical Sciences. 1996; 19(1):44–8.
Kurachi S, Hitomi Y, Furukawa M, et al. Role of intron I in expression of the human factor IX gene. J Biol Chem 1995; 270(10):5276–81.
Salie JP, Hirosawa S and Kurachi K . functional characterization of the 5′-regulatory region of human factor IX gene. J Biol Chem 1990; 265:7062–8.
Kozak K . The scanning model for translation: an update. J Cell Biol 1989; 108:229–41.
Chen BF, Hwang LH and Chen DS . Characterization of a biscistronic retroviral vector composed of the seine vesicular disease virus internal ribosome entry site. J Virol 1993; 67:2142–8.
Acknowledgements
The work was supported by National Natural Scientific Foundation, Shanghai Natural Scientific Foundation and National (863) high Technology Development program.
Author information
Authors and Affiliations
Additional information
*This paper is dedicated to Professor Lu Ji SHI on the 80th anniversary of his birthday
Rights and permissions
About this article
Cite this article
Zheng, B., Qiu, X., Tan, M. et al. Increment of hFIX expression with endogenous intron 1 in vitro. Cell Res 7, 21–29 (1997). https://doi.org/10.1038/cr.1997.3
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1997.3
Keywords
This article is cited by
-
Expression and regulation of hFIX minigene and cDNA driven by β-casein gene in mouse mammary gland
Science in China Series C: Life Sciences (1998)