Abstract
The undecapeptide substance P(SP) was shown to be intimately involved in both the structural and functional aspects of the anterior pituitary. Yet, in addition to its influences on hormonal secretion, SP may well possess more actions in this master gland. The present study was ftherefore aimed to investigate the effect of SP on the proliferation of rat anterior pituitary cells in primary culture. It was found that SP could dose-dependently increase the incorporation of tritiated thymidine (3H-TdR) into cultured anterior pituitary cells. Other mammalian tachykinins such as neurokinin A and neurokinin B had similar effect but to varying degrees. The equipotent analogue of SP, Norleucine11 – SP(Nle11-SP), also acted likewise, with its action antagonizable by spantide, a SP receptor blocker. To further characterize the nature of cells responsive to the challenge of SP, immunocytochemical staining against S-100 protein and some adenohypophyseal hormones was performed alone or plus autoradiography. The results showed that the percentage of S-100 protein-immunoreactive cells was apparently elevated by the addtion of Nle11-SP for 48 h, which indicates a preferential proliferation of folliculo-stellate cells under the regime. This was confirmed by increases in immunocytochemical or autoradiographical labelling indices of anterior pituitary cells treated similarly. Taken together, these results reveal that the trophic action of SP observed previously in other tissues is also present at least in cultured rat anterior pituitary cells, with responding cells being predominantly folliculo-stellate cells as typified by S-100 protein-immunoreactivity. Therefore, an intra-pituitary trophic action of SP in vivo could be anticipated.
Similar content being viewed by others
Introduction
There are increasing number of reports describing the multiple interactions between substance P(SP) and the anterior pituitary gland1, 2, 3, 4, 5, 6, 7, 8, 9. Firstly, the contents of SP in the anterior pituitary undergo dramatic fluctuations in accordance with the endocrine status of the body1. Immunocytochemical works further defined the localization of this neuropeptides in subsets of rat anterior pituitary cells such as thyrotropes, mammotropes and somatotropes2, 3. Being stored in the secretroy vesicles of these cells3, SP is synthesized de novo as verified by constitutive expression of preprotachykinin A mRNAs encoding the precursors of SP4. Moreover, the anterior pituitary cells bear specific membrane receptors of NK-1 type for SP5, 6, which justifies the widely observed yet diverse effects of SP on hormonal secretion from the anterior pituitary7.
In recent years, Ju et al. have convincingly identified the existence of SP-peptidergic nerve fibres in the anterior pituitary of several mammalian species8, 9. They even noticed synaptic structures between the nerve fibers and somatotropes or corticotropes in dogs under the immuno-electron microscopes9. These findings of not only challenge to the classical concept about the regulation of anterior pituitary functions, but also raise the question as to the exact roles SP plays in this pivotal gland. It has been described that SP could trophically stimulate neurite outgrowth10, smooth muscle cell11 as well as T lymphocyte proliferation12. Therefore, in addition to its secretion-modulating activity, SP may well possess other actions to be discovered in the anterior pituitary. Thereby, we attempted to explore the possible action of SP and related tachykinins on the in vitro proliferation of rat anterior pituitary cells as assessed by 3H-thymidine(3H-TdR) incorporation, immunocytochemical or autoradiographic labelling with sepcial reference to folliculostellate cells using the non-hormonal marker of S-100 protein immunostaining.
Material and Methods
Male Sprague-Dawley rats weighing 180-230 g were obained from the Lab Animal Center of this University. They were maintained in a constant environment (22 ± 3 °C, natural light cycle) with Lab chow and tap water ad libitum. SP, Norleucine11-SP (Nle11- SP), neurokinin A, neurokinin B and spantide [D-Arg1, D-Trp7-9, Leu11]-SP were all from Sigma Co., USA. TRH and LHRH were purchased from Shanghai Institute of Biochemistry, Academia Sinica. 3H-TdR(22 Ci/mM) was provided by Shanghai Institute of Atomic Energy. Other chemicals were all of analytical grade.
Cell culture
The method of cell preparation was basically adopted from previous reports13, 14. Briefly, rats were anaesthetized with phenobarbital i.p. and their anterior pituitaries were aseptically removed and placed in PBSA (NaCl 8.00 g, KCl 0.20 g, KH2PO4 0.20 g, Na2HPO4 .12H2O 2.88 g, BSA 1 g, penicillin 10,000 IU and streptomycin 10 mg per liter). After rinsing for 4 times they were chopped into small clumps (1 mm3) and washed again in PBSA. Tissues were then digested with 0.1% Trypsin-PBSA at 37 °C for 20 min in a metabolic shaker (100 rpm) followed by trituration with a pipetman. This procedure was repeated 1-2 times when necessary and the pooled cell suspension was spun at 200 × g for 10 min. The resulting cell pellet was suspended in DMEM-10% NCS (containing NaHCO3 33 mM, HEPES 11 mM, penicillin 100,000 IU, streptomycin 10 mg and newborn calf serum 100 ml per liter) and filtered with a nylon mesh (bore size 254 μ m). Cell density was calculated with a Neubaur hemocytometer and their viability assayed by trypan blue exclusion test. Cells were then seeded onto 96 well plates (Linbro) in densities of 1-2 × 105 cells/well and final volume of 200 μl. they were cultured in 5% CO2 at 37 °C with medium replaced on the following day.
3H-TdR incorporation assay
24 h after the addition of various reagents or vehicle(PBSA) into culture wells, cells were exposed to 3H-TdR (1 μ Ci/well, 22 Ci/mM) for another day. Upon brief trypsinization (0.25 % trypsin, 10 min), cells were collected with a multi-channel cell harvester (ZT-III, Zhejiang) onto glass fiber filters (G49). The dried filter slips were immersed in scintillation fluid (TP 4 g, POPOP 0.1 g, alcohol 20 ml and xylene added to 1,000 ml) and counted for cpm in FJ-2105 liquid scintillation counter (262, Xian).
Immunocytochemistry
For immunostaining, freshly dispersed cells were resuspended in PBSA and carefully seeded onto glass coverslips (1 × 1 cm2) in 50 μl containing 2–4 × 105 cells. They were incubated for 1 h at 37 °C and then gently immersed in 500 μl DMEM-10% NCS in either petri dishes (35 mm) or 24 well plates. After being cultured and treated with reagents for desired periods of time, the coverslips were quickly air-dried and fixed in 4% paraformaldehyde-0.1 M LPB (pH 7.4) for 30 min. Rinsed in 0.01 M PBS (pH 7.4) for 3x5 min, coverslips were treated with methanol-0.3 % H2O2 for 10 min to inactivate endogenous peroxidase activity, followed by rinsing again for 3∼5 min. The diluted primary antisera (rabbit anti-bovine S-100 protein: 1:4,000, anti-hGH 1:3000, anti-hACTH 1:4000, from Dakopatts and donated by Prof. Ju of this unit) were added to coverslips in a moist chamber at 4 °C for 48 h. Control staining was done by replacing the primary antibody with either antibody dilution buffer (0.01 M PBS containing 1% BSA and 0.02 % NaN3) or normal rabbit serum. Coverslips were exposed to biotinylated sheep anti-rabbit IgG antibody for 30 min at 37 °C, then to avidin-biotin conjugates (ABC immunostain kit, vector) at the same conditon and finally reacted with 0.05 % DAB-0.015 % H2O2 for 3-5 min, with regular washing in PBS for 3 × 5 min before each shift to next step. The enzymatic reaction was ended with tap water and the coverslips rinsed again in distilled water, counterstained with hematoxylin, dehydrated in alcohol, cleared in xylene and sealed with DPX on slides.
Immunocytochemistry and autoradiogrphy
Cells grown on coverslips were treated as designed and labelled with 3H-TdR for 48 h. They were then processed for immunostaining of ABC method as described above. Afterwards, the dried coverslips were smeared evenly with nuclear emulsion (Type-4, Beijing Institute of Atomic Energy) in darkroom. Upon drying they were placed into dark boxes with desiccant and exposed for 6 d at 4 °C. The emulsion-bearing coverslips were developed in Kodak D-19 b solution for 2–4 min (19 °C) and fixed routinely. When dried, the coverslips were mounted on slides with DPX and observed under microscope.
Data presentation
3H-TdR incorporation rates were expressed as percentages of control cpm values in oder to facilitate inter-batch comparison of data. The proportions of various cells were obtained by counting immunostained cells among the cultured anterior pituitary cell population. The indices of immunostained or autoradiographically labelled cells among all cells were calculated. These values were obtained by counting 500-1,000 cells at least twice per coverslip.
Results
Male rat anterior pituitary cells prepared by 0.1 % trypsin digestion plus mechanical dispersion were mostly single in round or polygonal shape, with viabilities over 90 % determined by trypan blue exclusion test. All types of anterior pituitary cells could be identified by immunocytochemical staining. Assessment of 3H-TdR incorporation rates and autoradiographic images revealed a low level of proliferating activity of anterior pituitary cells cultured under the present conditions. With this established system, we then added synthetic SP into culture medium in final concentrations of 10−13M -10−6 M for 48 h. The changes in 3H-thymidine incorporation rates are shown in Fig 1 in which clear dose-dependent responses can be seen. Repeated experiments gave similar results indicating an effective dose-range of SP starting from 10−10 M, which is nearly within the peak values of SP concentrations in blood plasma15.
Other tachykinins such as neurokinin A are also present in the anterior pituitary. It can be speculated that if the above action of SP really exists, then, neurokinin A may act similarly in this regard, for they share overlapping binding profiles for tachykinin receptors, though with different features. Comparison of the effects of SP, neurokinin A and neurikinin B was done by adding these agents into separate culture wells to the equal concentration of 10−9 M. It was found that SP and neurokinin A could potently stimulate the proliferatin of these cells in vitro after 2 days treatment time(Fig 2), as had been expected for.
To further verify the above facts, the equally potent substance P Nle11-SP16 was similarly administered. As shown in Fig 3, Nle11-SP again exhibited dose-related enhancement of 3H-TdR incorporation rates of cultured rat anterior pituitary cells, an action blockable by spantide (final concentration 10−7 M), a potent blocker of SP receptor.
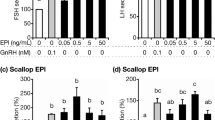
Nle11-SP was even compared with equimolar concentrations of TRH and LHRH, two factors with known mitogenic activities in the anterior pituitary both in vivo and in vitro. Likewise, they all stimulated the incorporation of 3H-TdR into cultured rat anterior pituitary cells but to varying degrees(Fig 4), with the former being less potent. Moreover, greater increases in 3H-TdR incorporation were seen in Nle11-SP and TRH co-added group, suggesting that their target cells might differ but overlap or their actions were simply additive.
To characterize the nature of anterior pituitary cells responsive to the stimulation by SP, Nle11-SP (10−8 M) treated and untreated cells grown on small glass coverslips were immunocytochemically stained with antibodies against hGH, hACTH or S-100 protein respectively with the percentages of positive cells enumerated. Only S-100 protein-immunoreactive cells, namely folliculo-stellate cells as shown in Fig 5, showed marked increases in their numbers after such treatment. This was confirmed by concomitant increases in the labelling indices of autoradigraphy of cells incorporating 3H-TdR under the influence of Nle11-Sp (Fig 6). In addition, we successfully performed immnuocytochemistry (ABC method) plus autoradiography of cells grown on small coverslips indicating the presences of 3H-TdR labelled cells immunoreactive to S-100 protein(folliculo-stellate cells) under similar treatment (Fig 7b). These facts add to the soundness of SP actions enhancing the proliferation of cultured rat enterior pituitary cells, especially folliculo-stellate cells.
a. S-100 protein-immunostaining of cultured rat anterior pituitary cells by the ABC method. Cells were grown on small coverslips for 48 h.
b. Images of S-100 protein-immunoreactive (arrow head) cells also positively labelled with 3H-TdR under the treatment of Nle11-SP (10−8 M) for 48 h (arrows indicating intense silver grans). Note the slender shape of cells.
a: × 260, b: × 450,
Discussion
The use of primary cell culture for the study of anterior pituitary cell proliferation has been repeatedly adopted which yielded much information about a host of substances capable of influencing this process (see review 17). In the present work, we chose to apply 3H-TdR incorppration test to reflect changes in DNA synthesis rate as well as immunocytochemical and/or autoradiographical techniques to reveal percentages of labelled cell identities in anterior pituitary cell culture. Both parameters gave consistent results demonstraing the proliferative effect of SP and related mammalian tachykinins on certain subsets of cultured anterior pituitary cell population. It may be argued that active proliferation of fibroblasts might constitute the responses observed here, as stated by Billestrup et a118. However, their report had not fully characterized the nature of fibroblasts in the presence of fetal calf serum, perhaps their conclusions were mainly based on morphological appearance of such cells. On the other hand, Childs et al19 described cultures of enriched rat corticotropes which displayed either flattened and pleomorphic countours of stellates shapes with multipe processes, features resembling those of fibroblasts. In this study we had ommitted fetal calf serum in culture medium to decrease fibroblast contamination. Most of flattened, stellate or spindle-shaped cells were positively stained against S-100 protein, a specific marker of folliculo-stellate cells20. Therefore, we have confidence in the reliability of our results to be free from major influences of fibroblasts, even if they are really exist.
Several reasons may account for the increased percentages of S-100 proteinimmunoreative cell under the stimulation of SP analogue-Nle11-SP: a) cell division accelerated; b) other cell types transform into folliculo stellate cells; c) changes in cell proportion due to cell death; d) induced expression of S-100 protein in other subsets of cells and e) false positivity in immunostaining. Up to now, no report is available which favors the second explanation. The third possibility is also less likely, for Wilfinger et al13 had observed no apparent changes in anterior pituitary cell proportions following enzymatic treatment and mechanical dispersion and we had noticed that the absolute numbers of S-100 protein-immunoreactive cells were obviously increased in the treatment group. Present evidence does not support the fourth claim but cannot fully exclude the possibility. Yet many considered S-100 protein to be restricted to folliculo-stellate cells in the anterior pituitary23. Lastly, the antisera to S-100 protein and others were well characterized and control staining with normal rabbit serum replacing the primary antibody was negative. Therefore, we think it is safe to conclude that enhanced cell division resulted in increased proportion of folliculo-stellate cells in anterior pituitary cell culture under the action of SP analogue. In fact we had observed a stimulation action of SP on the secretion from cultured anterior pituitary cells of interleukin 6 (IL-6) (unpublished results), which is most probably produced by folliculo-stellate cells, a strong indication in favour of our findings.
The trophic effects of SP were well documented on a variety of cells such as T lymphocytes11, smooth muscle cells12, submandibular gland cells and connective tissue cells. Our results further supplement this list with rat anterior pituitary cells. This in vitro impact of SP may have physiological significance in vivo, since SP could reach the anterior pituitary via hypophyseal portal vessels or from peptidergic nerve fibers innervating the gland[S], in addition to its synthesis and storage in thyrotropes2, mammotropes2 and somatotropes3. Moreover, the presence of SP receptors on the anterior pituitary was confirmed by serveral groups of investigators. They were shown to be of NK-1 type and act by hydrolysing polyphosphoino isitides in a GTP-dependent manner. Particularly, the effective concentrations of SP in this work coincide well with Kd values of these receptors5. However, the cellular distribution of SP receptors is not clear as well as the pattern of receptor subtype expression. The preferential proliferation of folliculo-stellate cells induced by SP analogue suggests nonhomogenous receptor distribution among cell subsets, as also revealed by discrepant influences of SP on various hormone secretion from the anterior pituitary. It may also implicate different proliferative capacity of distinct subsets of, and different cell cycles of, cultured anterior pituitary cells, a question demanding for deeper investigation.
Of all the anterior pituitary cells, the folliculo-stellate cells remain in search of a function23. They are characterized by their stellate shape and follicular organization. Beisdes, they lack secretory granules but posses elonagted nuclei and numerous mitochondria as well as microfilaments in their slender and tortous processes. Many speculations were raised about the role(s) they might play: support and nutrition, contractility and motility, phagocytosis, potential as stem cells and control of hormone secretion21, 23. Moreover, folliculo-stellate cells were found to secrete IL-622, basic FGF24 and vascular endothelial growth factor (VEGF)25. Thus they may be much more important and complicated than imagined. Tile recent description about the intimacy of folliculo-stellate cells with SP-immunoreactive nerve fibers9 can be regarded as one but not the Sole pathway whereby the trophic effect of SP stated above may occur in vivo in the anterior pituitary. Supportive clue is that endocrine mainipulation often results in morphological changes of folliculostellate cells21, 26 as well as SP contents in the anterior pituitary[l]. Further, SP could enhance tile secretion of IL-6 from cultured anterior pituitary cells of the rat (unpublished observation), and action most likely exerted on folliculo-stellate cells. It thus can be suspected that these cells may be physiologically controlled by SP in a paracrine or neurorine fashion or both, therefore performing many crucial fuctions in the organ, a problem worthy of further study.
In summary, SP and other tachykinins were found to be mitogenic to rat anterior pituitary cells in primary culture, stimulating the proliferation of certain cell subsets, especially folliculo-stellate cells.
References
Jones PM, O'halloran DJ, Ghatei MA, Domin J, Bloom SR . The influence of adrenal hormone status on neuroendocrine peptides in the rat anterior pituitary gland. J Endocrinol 1990; 127:437–44.
Brown ER, Roth KA, Krause JE . Sexually dimorphic distribution of substance P in specific anterior pituitary cell populations. Proc Natl Acad Sci USA 1991; 88:1222–6.
Morel G, Chayvialle JA, Kerdelhue B, Dubois PM . Ultrastructural evidence for endogeneous substance P-like immunoreactivity in the rat pituitary gland. Neuroendocrinology 1982; 35:86–92.
Brown ER, Harlan RE, Krause JE . Gonadal steroid regulation of substance P (SP) and Sp-encoding mRNAs in the rat anterior pituitary and hypothalamus. Endocrinology 1990; 126:330–40.
larsen P J, Mikkelsen JD, Mau SE, Saermark T . Binding and internalization of an iodinated substance P analogue by cultured anterior pituitary. Mol Cell Endocrinol 1989; 65:91–101.
Mau SE, Saermark T . Substance P stimulation of polyphosphoinositide hydrolysis in rat anterior pituitary membranes involves a GTP-depeudent mechanism. J Endocrinoal 1991; 130: 63–70.
Arisawa M, Snyder GD, Yu NH, DePalatis LR, Ho RH, McCann SM . Physiologically significant inhibitory hypothalamic action of substance P on prolactin release in the male rat. Neuroen-docrinol 1992; 52:22–7.
Liu SJ, Ju G . Substance P-like immunoreactive nerve fibers in the pars distalis of the adenohypophysis of macaque monkeys. Neurosci Lett 1988; 94:1–4.
Ju G, Zhang X . An electron microscopic study on substance P-like immunoreactive nerve fibers in the pars distalis of the adnohypophysis in the dog. Neuroscience 1990; 38:503–13.
Iwasaki Y, Kinoshita M, Ikeda K, Takamiya K, Shiojima T . Trophic effect of various neuropeptides on the cultured ventral spinal cord of rat embryo. Neurosci Lett 1989; 101:316–20.
Payan DG . Receptor-mediated mitogenic effects of substance P on cultured smooth muscle cells. Biochem Biophys Res Comm 1985; 130:104–9.
Payan DG, Brewster DR, Goetzl EJ . Specific stimulation of human T lymphocytes by substance P. J Immunol 1983; 131:1613–5.
Wilfinger WW, Larsen W J, Downs TR, Wilbur DL . An in vitro model for studies of intercellular communication in cultured rat anterior pituitary cells. Tissue and Cell 1984; 16:483–97.
Smith PF, Luque EH, Neill JD . Detection and measurement of secretion from individual neuroendocrine cells using a reverse hemolytic plaque assay. Methods Enzymol 1986; 124:443–65.
Pernow B . Substance P. Pharmacol Review 1983; 35:85–141.
Echer E, Couture R, Champagne G, Mizrahi J, Regoli D . Synthesis and biological activities of photoaffinity labelling analogues of substance P. J Med Chem 1982; 25:470–5.
Zhang WH, Zhu YL, Wang FZ . The regulation of anteror pituitary cell proliferation. Chinese J Cell Biol 1993; 15: 160–64(in Chinese).
Billestrup N, Swanson LW, Vale W, Verma IM . Growth hormonereleasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci USA 1986; 83:6854–7.
Childs GV, Lloyd J, Unabia G, Rougeau D . Growth and secretory responses of enriched populations of corticotropes. Endocrinology 1989; 125:2540–9.
Nakajima T, Yamaguchi H, Takamashi K . S-100 protein in folliculo-stellate cells of the rat anterior lobe. Brain Res 1980; 191: 523–31.
Vila-Porcile E, Olivier L . The problem of folliculostellate cells in the anterior pituitary gland. In: Motta PM ed. Ultrastructure of endocrine cells and tissues. Martinus Ni jhoff Publishers, Boston 1984:64–76.
Vankelecom H, Carmeliet P, van Damme J, Billiau A, Denef C . Production of interleukin-6 by folliculo-stellate cells of the anterior pituitary gland in a histotypic cell aggregate culture system. Neuroendocrinology 1989; 49:102–6.
Perryman EK . Folliculo-stellate cells of the pituitary gland what roles do these star-shaped cells play. Bioscience 1989; 39:81–8.
Ferrara N, Schweigerer L, Neufeld G, Mitchell R, Gospodarowicz D . Pituitary folliculo cells produce fibroblast growth factor. Proc Natl Acad Sci USA 1987; 84: 5773–7.
Ferrara N, Henzel WJ . Pituitary folliculo cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Comm 1989; 161:851–8.
Sbarbati A, Zancanaro C, Barbatelli G, Cinti S, Osculati F . Ultrastructure of pituitary folliculo-stellate cells of lactating rat during treatment with 2-bromo-alpha-ergocriptine. Tissue and Cell 1989; 121:841–7.
Acknowledgements
We wish to thank Prof. Ju G for his kind gift of antisera used in the study. Our thanks also go to Prof. Jin BQ and Ms. Li YR for their technical assistance. This work was supported by National Natural Science Foundation of China and in part by a Young Investigator's Research Grant of PLA to Wanhui Zhang.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, W., Zhu, Y., Wang, F. et al. Substance P enhances the proliferation of rat anterior pituitary cells in vitro. Cell Res 5, 197–207 (1995). https://doi.org/10.1038/cr.1995.19
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1995.19