Abstract
In present study, we studied the effect of all-trans retinoic acid (ATRA) and dimethylsulfoxide (DMSO) on the induction of apoptosis in HL-60 cell line. Based on morphological changes by Hochest 33342 staining and identification of internucleosomal DNA cleavage by gel electrophoresis, we observed aberrant nuclear chromatin condensation and ladder-like pattern of DNA degradation. Using Flow Cytometric method, we found sub-G1 peak in RA-treated HL-60 cells starting 5 to 6 d after the initiation of the treatment. However, such an obvious apoptotic peak was not identified in DMSO-differentiated cells. Combining the research accomplished before, our study approves further that apoptosis could be a common mode of death of terminally differentiated HL-60 cells.
Similar content being viewed by others
Introduction
The Human Leukemic HL-60 cells have been used extensively as a model to study the control of cell proliferation and differentiation. These cells can be induced to differentiate into granulocytes by treatment with retinoic acid (RA)1 or dimethylsulfoxide(DMSO)2. From 1986, all-trans retinoic acid (ATRA) has been used as a clinical antitumor agent in the treatment for patients with acute promyelocytic leukemia(APL) in China and proved to be successful on the basis of a high rate(above 80%) of complete remission(CR) in previoulsy untreated or relapsed patients with APL3. In the present study, we showed that a remarkable apoptotic cell death had been triggered after treatment of HL-60 cells with 1 μmol/L RA or 1.25% DMSO for 6 d. Therefore, our study approves that apoptosis could be the common mode of death of terminally differentiated HL-60 cells, and also suggests a possible mechanism of apoptosis in treatment of some APL patients with ATRA.
Materials and methods
Cells and cell culture
The HL-60 cells were maintained in RPMI1640 medium (Gibco) supplemented with 10% calf serum and 2 nmol/L glutaminein at 37°C in a humidified atmosphere of 5% CO2 in air.
Growth inhibitory curve
HL-60 cells were suspended at a concentration of 7.5 × 104 cells/ ml in RPMI1640 and treated with 1 μmol /L RA and 1.25% DMSO respectively for 6 d. Cell viability was determined using trypan blue exclusion test.
Hochest 33342 staining
Cells untreated or treated with RA or DMSO for 6 d were rinsed in PBS and cytocentrifuged on slides. Then cells were stained with Hochest 33342 at a final concentration of 10 μmol/L for 15 min in the dark. Photographs were taken under fluorescent microscope by ultraviolet lighL
Analysis of DNA fragmentation by agarose gel electrophoresis
Cellular DNA was extracted as described previously4. Electrophoresis was performed in 1.8% agrose gel. After electrophoresis, DNA was visualized by ethidium bromide staining.
Analysis of DNA content by flow Cytometric method
Cells were rinsed in PBS containing 0.1% Triton X-100 and resuspended in 1 ml PBS containing 5 μg/ml PI and 0.1% RNase (Sigma). Red(DNA) fluorescence of individual cells was measured with a FAC Scan Flow Cytometer.
Results and Discussion
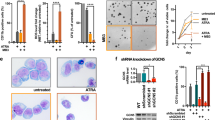
HL-60 cells, a leukemic promyelocytic cell line can be induced to differentiate into neutrophils or granulocytes by several kinds of chemicals. Untreated human leukemic HL-60 cells grew quickly as single cell suspension culture. From growth inhibitory curve(Fig 1), we have found an obvious effect of both RA (1 μ mol/L) and DMSO (1.25%) on inhibiting HL-60 cell proliferation after 2 d. The result of trypan blue exclusion test showed that above 97% untreated HL-60 cells were still alive on the sixth day, while part of cells treated with either RA or DMSO were dyed by trypan blue. Their survival rate in this exclusion test was 71% and 79% respectively.
To study nuclear organization in treated cells, Hochest 33342 staining was performed. Fig 2 showed that there was a highly chromatin condensation in HL-60 cells treated with both RA and DMSO. Comparing the effects of two drugs on aberrant chromatin organization, we have found that, in RA-treated cells, nuclear fragmentation condensed to several highly compact speckles while in DMSO-treated cells, chromatin seemed to break into small pieces.
The discontinuous DNA degradation, assessed as the presence of mono- and oligonucleosomal fragmentation, is considered to be hallmark of apoptosis. After treatment of HL-60 cells with RA or DMSO for 6 d, a remarkable ladder-like electrophoretic pattern of DNA was obviously visualized under ultraviolet light after staining with 5 μg/ml ethidium bromide (Fig 3). However in untreated growing HL-60 cells, there was no sign of DNA degradation.
Flow Cytometric method, for certain antitumor agents but not all, is another sensitive and effective means to examine DNA degradation. The analysis of DNA content of RA-treated cells (Fig 4) showed a sub-G1 peak (Ap peak) in RA-treated HL-60 cells for 5 and 6 days. However, such an obvious apoptotic peak was not identified by FCM in DMSO-treated cells. This result indicated a different pathway of apoptosis may exist between ATRA and DMSO. Besides, it suggested that FCM could be used as a simple method in clinical trials to examine apoptosis triggered by ATRA.
Enhancement of tumor cell responsiveness by apoptosis is being considered as one of the factors that may improve the effectiveness of antitumor therapy. Therefore, new strategies may involve combination of drugs or treatments that potentiate the apoptotic response. Most published studies focused on the induction of apoptosis in undifferentiated and differentiated HL-60 cells by various physical and chemical stimuli, such as DNA topoisomerase I and II inhibitors 5, 6, etoposide camptothecin and other anticancer drugs7. Since differentiation agents are already used in clinical trials, their possible mechanism in terms of induction of apoptosis is of interest in oncology. Recently, it was reported that TPA induced DNA fragmentation in HL-60 cells which failed to attach early to the culture dish, but not in adherent differentiated cells. However, once these cells detached from the cultrre dish, they would undergo apoptosis in the end8. Besides, Calabresse et al found no apoptosis in APL cells in primary culture after ATRA treatment, but on the other hand, these cells could be induced to undergo apoptosis by etoposide, a topoisomerase II inhibitor, which indicated an alteration involved in ATRA pathway(s) of apoptosis in some primary cultured APL cells9. In our work, we observed a remarkable apoptosis in terminal differentiated HL-60 cells with treatment of RA or DMSO, assessed as chromatin condensation and nuclear fragmentation as well as ladder-like pattern of DNA degradation. Our results are consistent with Calabresse's suggestions that several signal transduction pathways of apoptosis can co-exist in one cell. What's more, the two processes of differentiation and apoptosis are both interrelated and different, therefore, induction of apoptosis can be caused after the treatment with differentiating agent during cell differentiation, which can be controlled by apoptotic proteins and those associated with cell survival9. The.studies on apoptosis of HL-60 cells induced by traditional antitumor agents such as RA and DMSO may provide a possible role of induction of apoptosis by ATRA in clinical treatment. Combining the prior research studies, our results here approved further that apoptosis could be the common mode of death of terminally differentiated HL-60 cells8.
References
Breitman TR, Selonick SE, Collins SJ . Proc Natl Acad Sci USA 1980; 77:2936–40.
Collins S J, Ruscetti FW, Gallagher RE, Gallo RC . Proc Natl Acad Sci USA 1978; 75:2458–62.
Sun GL, Huang YG, Chang XF, Jiang S, Zhang T . Chinese Journal of Hematology, 1992; 13:135–8.
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning (2nd ed.)Cold Spring Harbor Laboratory Press 1980;p464–7.
Lennon SV, Martin S J, Cotter TG . Cell Prolif 1991; 24:203.
Bertrand R, Sarang M, Jenkins J, Kerrigan D, Pommier Y . Cancer Res 1991; 51:6280–4.
Kaufmann SH . Cancer Res 1989; 49:5870–4.
Solary E, Bertrand R, Pommier Y . Leukemia 1994; 8:792–7.
Calabresse C, Ventarini L, Barbey S, et al. Blood 1994; 84:600a. (Abstract in Supplement)
Author information
Authors and Affiliations
Additional information
*This work was supported by the National Medical and Scientific Key Item Grant of “8.5 Plan”.
Rights and permissions
About this article
Cite this article
Sun, H., Wang, Y. Apoptosis of human leukemic HL-60 cells induced to differentiate by treatment with RA or DMSO. Cell Res 5, 181–186 (1995). https://doi.org/10.1038/cr.1995.17
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1995.17
Keywords
This article is cited by
-
A Computational Systems Biology Approach for Identifying Candidate Drugs for Repositioning for Cardiovascular Disease
Interdisciplinary Sciences: Computational Life Sciences (2018)