Abstract
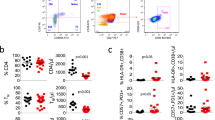
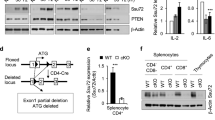
T-cells critically contribute to protection against Mycobacterium tuberculosis infection, and impaired T-cell responses can lead to disease progression. Pro-inflammatory and immunosuppressive cytokines affect T-cells, and fine-tuned regulation of cytokine signaling via the Jak/STAT signaling pathways is crucial for appropriate T-cell function. Constitutive STAT3 phosphorylation as a consequence of aberrant cytokine signaling has been described to occur in pathognomonic T-cell responses in inflammatory and autoimmune diseases. We characterized blood samples from tuberculosis patients (n=28) and healthy contacts (n=28) from Ghana for M. tuberculosis-specific T-cell responses, constitutive cytokine production, and SOCS3 and pSTAT3 expression. Lentiviral modulation of primary CD4+ T-cells was performed to determine the effects of SOCS3 on T-cell functions. T-cells from tuberculosis patients expressed higher levels of IL-10 and IL-6 and lower levels of T helper type (TH)17 cytokines after M. tuberculosis-specific stimulation compared to healthy contacts. In addition, tuberculosis patients had higher IL-10 and IL-6 levels in the supernatants of non-stimulated immune cells and plasma samples compared to healthy contacts. Notably, aberrant cytokine expression was accompanied by high constitutive pSTAT3 levels and SOCS3 expression in T-cells. Multivariate analysis identified an IL-6/IL-10 co-expression-based principal component in tuberculosis patients that correlated with high pSTAT3 levels. SOCS3 contributed to a regulatory component, and tuberculosis patients with high SOCS3 expression showed decreased TH1 cytokine expression and impaired IL-2-induced STAT5 phosphorylation. SOCS3 over-expression in primary CD4+ T-cells confirmed the SOCS3 inhibitory function on IL-2-induced STAT5 phosphorylation. We conclude that constitutive pSTAT3 and high SOCS3 expression are influential factors that indicate impaired T-cell functions in tuberculosis patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cooper AM, Flynn JL. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol 1995; 7: 512–516.
Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nature reviews Immunology 2011; 11: 343–354.
Chowdhury IH, Ahmed AM, Choudhuri S, Sen A, Hazra A, Pal NK et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Molecular immunology 2014; 62: 159–168.
Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B et al. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC infectious diseases 2013; 13: 13.
Masood KI, Rottenberg ME, Carow B, Rao N, Ashraf M, Hussain R et al. SOCS1 gene expression is increased in severe pulmonary tuberculosis. Scand J Immunol 2012; 76: 398–404.
Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010; 181: 734–742.
Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol 1999; 115: 110–113.
Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang AT et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol 2014; 151: 84–99.
Jones LL, Alli R, Li B, Geiger TL. Differential T Cell Cytokine Receptivity and Not Signal Quality Distinguishes IL-6 and IL-10 Signaling during Th17 Differentiation. J Immunol 2016; 196: 2973–2985.
Hirano T, Kishimoto T. Interleukin-6: possible implications in human diseases. Ric Clin Lab 1989; 19: 1–10.
Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol 2017.
Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 2006; 116: 1218–1222.
Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006; 36: 1357–1366.
Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol 2007; 4: 269–275.
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993; 75: 263–274.
Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 2007; 8: 1363–1371.
Parish IA, Marshall HD, Staron MM, Lang PA, Brustle A, Chen JH et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J Clin Invest 2014; 124: 3455–3468.
Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005; 79: 10514–10527.
Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007; 178: 2623–2629.
Carow B, Reuschl AK, Gavier-Widen D, Jenkins BJ, Ernst M, Yoshimura A et al. Critical and independent role for SOCS3 in either myeloid or T cells in resistance to Mycobacterium tuberculosis. PLoS pathogens 2013; 9: e1003442.
Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol 2003; 4: 546–550.
Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 2003; 170: 3263–3272.
Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem 2013; 288: 2986–2993.
Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Frontiers in Immunology 2014; 5: 58.
Banerjee A, Banks AS, Nawijn MC, Chen XP, Rothman PB. Cutting edge: Suppressor of cytokine signaling 3 inhibits activation of NFATp. J Immunol 2002; 168: 4277–4281.
Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem 2003; 278: 29752–29759.
Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS et al. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Molecular and Cellular Biology 1999; 19: 4980–4988.
Matsumoto A, Seki Y, Watanabe R, Hayashi K, Johnston JA, Harada Y et al. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. The Journal of experimental medicine 2003; 197: 425–436.
Rottenberg ME, Carow B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin Immunol 2014; 26: 518–532.
Jacobsen M, Repsilber D, Kleinsteuber K, Gutschmidt A, Schommer-Leitner S, Black G et al. Suppressor of cytokine signaling-3 is affected in T-cells from tuberculosisTB patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2011; 17: 1323–1331.
Lundtoft C, Afum-Adjei Awuah A, Rimpler J, Harling K, Nausch N, Kohns M et al. Aberrant plasma IL-7 and soluble IL-7 receptor levels indicate impaired T-cell response to IL-7 in human tuberculosis. PLoS pathogens 2017; 13: e1006425.
Isomaki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology (Oxford) 2015; 54: 1103–1113.
Kuuliala K, Kuuliala A, Koivuniemi R, Oksanen S, Hamalainen M, Moilanen E et al. Constitutive STAT3 Phosphorylation in Circulating CD4+ T Lymphocytes Associates with Disease Activity and Treatment Response in Recent-Onset Rheumatoid Arthritis. PLoS One 2015; 10: e0137385.
Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K et al. Mycobacterium tuberculosis-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. Cytokine 2008; 43: 143–148.
Schuck SD, Mueller H, Kunitz F, Neher A, Hoffmann H, Franken KL et al. Identification of T-cell antigens specific for latent mycobacterium tuberculosis infection. PLoS One 2009; 4: e5590.
Kleinsteuber K, Heesch K, Schattling S, Sander-Juelch C, Mock U, Riecken K et al. SOCS3 promotes interleukin-17 expression of human T cells. Blood 2012; 120: 4374–4382.
Sokal RR, RF. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman & Co Ltd. 1981.
Mistry R, Cliff JM, Clayton CL, Beyers N, Mohamed YS, Wilson PA et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. The Journal of infectious diseases 2007; 195: 357–365.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003; 4: 540–545.
Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol 2002; 168: 3181–3187.
Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 2015; 5: 17663.
Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C et al. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia 2005; 19: 209–213.
Brender C, Nielsen M, Kaltoft K, Mikkelsen G, Zhang Q, Wasik M et al. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood 2001; 97: 1056–1062.
Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem 2003; 278: 16777–16781.
Anderson AE, Pratt AG, Sedhom MA, Doran JP, Routledge C, Hargreaves B et al. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis 2016; 75: 466–473.
Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol 2005; 100: 64–72.
Nieminen JK, Niemi M, Sipponen T, Salo HM, Klemetti P, Farkkila M et al. Dendritic cells from Crohn's disease patients show aberrant STAT1 and STAT3 signaling. PLoS One 2013; 8: e70738.
Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM et al. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol 2015; 194: 3422–3431.
Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets 2008; 9: 369–374.
Pratt AG, Swan DC, Richardson S, Wilson G, Hilkens CM, Young DA et al. A CD4 T cell gene signature for early rheumatoid arthritis implicates interleukin 6-mediated STAT3 signalling, particularly in anti-citrullinated peptide antibody-negative disease. Ann Rheum Dis 2012; 71: 1374–1381.
Bandaru A, Devalraju KP, Paidipally P, Dhiman R, Venkatasubramanian S, Barnes PF et al. Phosphorylated STAT3 and PD-1 regulate IL-17 production and IL-23 receptor expression in Mycobacterium tuberculosis infection. European Journal of Immunology 2014; 44: 2013–2024.
Jung BG, Wang X, Yi N, Ma J, Turner J, Samten B. Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3. Sci Rep 2017; 7: 40984.
Queval CJ, Song OR, Deboosere N, Delorme V, Debrie AS, Iantomasi R et al. STAT3 Represses Nitric Oxide Synthesis in Human Macrophages upon Mycobacterium tuberculosis Infection. Sci Rep 2016; 6: 29297.
Rojas JM, Avia M, Martin V, Sevilla N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol Res. 2017; 2017: 6104054.
Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A et al. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol 1999; 92: 224–234.
Cyktor JC, Carruthers B, Beamer GL, Turner J. Clonal expansions of CD8+ T cells with IL-10 secreting capacity occur during chronic Mycobacterium tuberculosis infection. PLoS One 2013; 8: e58612.
Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 2000; 105: 1317–1325.
Pinheiro RO, de Oliveira EB, Dos Santos G, Sperandio da Silva GM, de Andrade Silva BJ, Teles RM et al. Different immunosuppressive mechanisms in multi-drug-resistant tuberculosis and non-tuberculous mycobacteria patients. Clin Exp Immunol 2013; 171: 210–219.
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 2007; 8: 1390–1397.
Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. The Journal of experimental medicine 2009; 206: 1009–1017.
Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 2003; 4: 551–556.
We would like to thank all study participants, study nurses, and physicians who made this study possible. This study was supported by the German Research Foundation (DFG, JA 1479/5-1) to N Nausch, E. Owusu-Dabo, and M. Jacobsen. K. Harling were supported by the ‘Hedwig und Waldemar Hort Stipendienstiftung’. E. Adankwah and M. Jacobsen were supported by the Manchot graduate school ‘Molecules of Infection (MOI)-3’.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Harling, K., Adankwah, E., Güler, A. et al. Constitutive STAT3 phosphorylation and IL-6/IL-10 co-expression are associated with impaired T-cell function in tuberculosis patients. Cell Mol Immunol 16, 275–287 (2019). https://doi.org/10.1038/cmi.2018.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2018.5
Keywords
This article is cited by
-
Differences in PPD- and mitogen-induced T-cell activation marker expression characterize immunopathology in acute tuberculosis patients
European Journal of Clinical Microbiology & Infectious Diseases (2024)
-
Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial
Stem Cell Research & Therapy (2023)
-
Cytokine upsurge among drug-resistant tuberculosis endorse the signatures of hyper inflammation and disease severity
Scientific Reports (2023)
-
Impaired T-cell response to phytohemagglutinin (PHA) in tuberculosis patients is associated with high IL-6 plasma levels and normalizes early during anti-mycobacterial treatment
Infection (2023)
-
Plasma cytokine levels characterize disease pathogenesis and treatment response in tuberculosis patients
Infection (2023)