Abstract
Autophagy and immunity share the property of being auto-protective for the organism. Autophagy is an important degradation pathway that buffers nutrient deprivation by recycling macromolecules in organisms from yeast to man. Perturbations in autophagy are associated with inflammation and cancer development. Emerging studies have characterized the molecular details regarding how autophagy is controlled by immune cells. Among these, dendritic cells (DCs) are one of the most potent professional antigen-presenting cells critical for the activation of naïve T cells to maintain immune tolerance and drive protective immunity to infection and cancer. DCs undergo functional maturation that can either lead to an immunostimulatory phenotype, as in the context of infection, or to a tolerogenic phenotype associated with immunosuppression to self-antigens, as well as to cancer. An increasing number of recent studies has characterized the involvement of autophagy in DC functions in various physiological and pathological contexts. Here, we provide a comprehensive review of these outcomes and discuss the limitation of the models used and the forefront of the knowledge concerning the crosstalk between autophagy and DC biology.
Similar content being viewed by others
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells critical for the interdependence between the innate and adaptive immune systems. Their distinctive ability to prime naïve T cells plays key roles in protective immunity to infection and cancer as well as in maintaining immune homeostasis and tolerance. These properties are because, among all antigen presenting cells, DCs stand out in particle uptake and antigen presentation. These processes require high levels of endocytic and lysosomal activity that are tightly linked to macroautophagy. Several reports have highlighted during the last decade the modulation of DC functions by macroautophagy. This catabolic process is evolutionarily conserved and shares with immunity the property of being auto-protective for the organism by regulating the cellular stress response in general and, particularly, the cellular functions of immune cells. Starvation is considered one of the most potent inducers of macroautophagy in eukaryotes. In immune cells, such as DCs, in addition to starvation, several environmental stress-related stimuli interact with autophagic pathways to drive their immune functions.
Types of macroautophagy
At least three different types of canonical autophagy coexist in DCs: (i) microautophagy that involves the capture of cytosolic components by lysosomes through various modifications on their membranes; (ii) chaperone-mediated autophagy by which proteins holding KFERQ-like motifs translocate into the lysosomal lumen; and (iii) macroautophagy that leads to bulk lysosomal degradation of the cytosol via double-membrane organelles called autophagosomes.1 Because more attention has been given to macroautophagy in DCs and because the evidence of active microautophagy2 and chaperone-mediated autophagy3 in DCs were shown only indirectly in primary cell cultures, we will focus here on the regulation of the functions of DCs by macroautophagy that we will call autophagy.
Molecular mechanisms of autophagy in DCs
Autophagy is orchestrated by the dynamic assembly and interactions of up to 31 autophagy-related proteins (ATGs) identified to date. The detailed functions of these genes have been widely discussed elsewhere;4 herein, we will only provide a brief summary of those proteins reported to be associated with DC functions. They include a complex formed by UNC-51-like kinase 1 (ULK1), ATG13, focal adhesion kinase family interacting protein of 200 kD (FIP200) and ATG101 involved in the initiation of the phagophore, the first nascent membrane during autophagosome formation. The elongation of the phagophore is controlled by two complexes. The first is a ubiquitin-like conjugation system formed by the E1-like enzyme ATG7-mediated binding of ATG12 and ATG5, which later oligomerize with ATG16L under the control of the E2-like enzyme ATG10. The second is formed by Beclin 1, phosphatidylinositol 3-kinase class III (PI3K), p150 and ATG14, simply called the class III PI3K complex. Beclin 1 is recruited to this complex after its dissociation from BCL-2 under the effect of some autophagy inducers such as High-mobility group box 1 (HMGB1). Ultimately, MAP1LC3B binds covalently to phosphatidylethanolamine to form lipidated LC3-II on the autophagosomal membrane after a series of ubiquitin-like conjugation reactions catalyzed by the E1-like enzyme ATG7 and E2-like enzyme ATG3 (Figure 1). Notably, LC3 associates as well to phagosomes during phagocytosis, which constitutes an LC3-associated phagocytosis (LAP) pathway that is considered non-canonical autophagy.5 The LAP relevance in DC biology has been reviewed and highlighted in several reports.5,6 Here, we will focus on the involvement of canonical autophagy in DC functions.
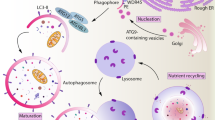
Scheme of the autophagic machinery described in DCs. The dynamic assembly and interactions of ATG proteins are highlighted: the ULK1 complex, class III PI3K complex and ubiquitin-like conjugation systems for ATG5-ATG12-ATG16 complex and the recruitment of LC3 to autophagosomal membranes. The thick arrows indicate trafficking routes, and the thin arrows indicate enzymatic reactions.
Autophagy was shown to be involved in DC functions at several levels: DC maturation, triggers of DC maturation such as Toll-like receptor (TLR) stimulation, antigen presentation, cytokine production, DC migration and maturation and T-cell activation. Herein, we will review, consecutively, the role of autophagy and ATG proteins in these functional properties of DCs (Figure 2 and Table 1).
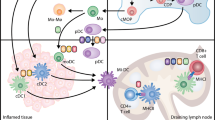
Scheme describing the involvement of ATG genes in the functional aspects of DC maturation. Each step of DC functional maturation is described with the ATG proteins reported to be involved in their regulation. See Table 1 for references. LN: lymph node; MHC-II: major histocompatibility complex class II; PAMP: pathogen-associated molecular patterns; TCR: T-cell receptor; TLR: Toll-like receptor.
Role of autophagy in the phenotypic maturation of DCs
DCs are distributed throughout the tissues of the body where various stimuli can induce their maturation. DCs endure two states of maturation morphologically, phenotypic and functional. The change from the latent (tolerogenic) state to the activated one is associated with functional maturation and an immunostimulatory phenotype. This type of maturation occurs in response to DC activation by pathogen-associated molecular patterns (PAMPs), the most well-characterized being the TLRs. It involves the up-regulation of antigen presentation and acquisition of the migratory potential, enabling cells to migrate to secondary lymphoid tissues and interact with naïve T cells and trigger effector T-cell responses. A second state of DC maturation, less characterized, occurs in the steady-state where the up-regulation of major histocompatibility complex class-II (MHC-II), co-stimulatory molecules and a series of other genes is associated with DC migration to draining lymph nodes to subsequently trigger regulatory T-cell responses to maintain immune homeostasis and tolerance.27
Various reports have associated autophagy with one or several features of the complex DC maturational program as summarized below.
During DC maturation, the Hsc/Hsp70 co-chaperone network was shown to control the transient formation of DC aggresome-like-induced structures (DALIS) by modulating their autophagic degradation.28 In human DCs infected with Bacillus Calmette-Guerin, autophagy stimulation by starvation was reported to up-regulate the expression of CD86 and HLA-DR, as well as the secretion of IL-10 and IL-6.29 In the context of hapten-induced maturation of DCs, the skin sensitizer 1-fluoro-2,4-dinitrobenzene was shown to modulate, via the PERK-eIFα-ATF4 pathway, the maturation of DC-like THP-1 cells through up-regulating the transcription of IL-8 while blocking the transcription of CD86 and pro-inflammatory cytokines IL-1β, IL-12β, and CXL10. This was associated with an ATF4-dependent induction of the transcription of autophagy-related genes MAP1LC3B and ATG3.30 Furthermore, mice with ATG16L1 deficiency exert an increased expression of costimulatory molecules CD86 and CD80 in the DCs of a mouse model of allogeneic hematopoietic stem cell transplantation. This phenotype was probably due to decreased amounts of A20 observed in these DCs, which is a negative regulator of their maturation.22
DC maturation during viral infection has also been associated with autophagy. For example, blockade of autophagy in bone marrow-derived DCs (BMDCs) infected with respiratory syncytial virus (RSV) inhibited their maturation as assessed by the expression of MHC-II and co-stimulatory molecules CD40, CD80, and CD86.17 Another example is the effects of autophagy inhibition in plasmacytoid DCs (pDCs) infected with paramyxovirus Simian Virus 5 SV5, where a decrease in CD80 expression was observed.31
Autophagy can lead to reduced antigen presentation and DC maturation during cancer chemothrapy.32 Regulatory T cells (Tregs) also reduce the immunogenic maturation of DCs through the inhibition of their autophagic machinery in DCs.33 Autophagy has been associated with the tolerogenic maturation of DCs and was suggested to contribute to the enhanced allograft survival during transplantation.34
Overall, most reports with few exceptions support an inhibitory role for autophagy in the immunogenic maturation of DCs and a positive role in their tolerogenic maturation. However, whether there is a specific maturation signature under the control of autophagy in DCs or in selected DC subsets has yet to be revealed.
Autophagy and functional properties of DCs
DC migration and autophagy
DCs are strategically situated throughout the organism to respond to invading pathogens in tissues and maintain tolerance in the steady state. Their migration is an important feature for their recruitment to sites of challenge and their traffic to secondary lymphoid organs where they communicate with lymphocytes to coordinate adaptive immune responses. Autophagy was very recently linked to some of the molecular trafficking signals that govern the migration of DCs, as discussed below.
The migration of DCs was found to be reduced when infected with Orientia tsutsugamushi that, on the other hand, was shown to activate autophagy in BMDCs.21 A more direct role of autophagy in DC migration was provided by ATG16-deficient DCs that showed reduced migration to lymph nodes and increased cellular adhesion. This phenotype was due to a defect in DC cytoskeletal modulation through the loss of filopodia, altered podosome distribution, and increased membrane ruffling. Using thiopurines that switch-off the hyper-activation of the small Rho GTPase Rac1 occurring in these autophagy-deficient DCs, the migratory potential of these DCs was successfully restored.21 Given that thiopurines are commonly used drugs in the therapy of Crohn's disease and a genetic risk factor exclusively associated with Crohn's disease is a variant in ATG16L1 that reduces autophagy, targeting autophagic pathway nodes may sustain potential drugs to improve DC migration in a disease context where their involvement becomes crucial. Therefore, autophagy in DCs seems to control their migration through the modulation of their cytoskeleton.
TLR signaling and autophagy
TLRs are in either plasma membranes or endosomal-lysosomal membranes. Both types of membranes are involved in autophagic vesicle trafficking.35 Thus, it is not surprising that the pathways that emanate from the activation of TLRs are associated with autophagy alteration and vice versa.
The first evidence of autophagy crosstalk with TLR signaling was shown in primary human and murine macrophages.36 Next, autophagy involvement in the TLR-mediated immune response in DCs was provided by a study showing the requirement of autophagy in the recognition of viral PAMPs by TLR7 and their transport to the lysosomes in pDCs that uniquely express this TLR.8 Similar observations reported a dramatic drop in the TLR4- and TLR8-mediated responses in autophagy-deficient DCs stimulated with lipopolysaccharide (LPS) and ssRNA.9 This upstream regulation of TLR signaling by autophagy was very recently verified in a disease context where a loss of autophagy in DCs slowed a TLR7-mediated model of autoimmunity akin to systemic lupus erythematosus.10
Autophagy not only acts upstream of TLR signaling but can also be regulated by TLR stimulation. For example, TLR4 stimulation in DCs was recently shown to inhibit autophagy as a consequence of mTORC1 activation.37 However, combinatorial stimulation of TLR4 and nucleotide-binding oligomerization domain-containing-2 (NOD2) in DCs was shown to activate autophagy,38 suggesting that the previously reported NOD2 impact on autophagy activation39 is predominant over TLR4 stimulation.
Taken together, while autophagy was shown to impact downstream signaling through some TLRs (TLR-4, -7 and -8), its regulation by TLR signaling is less clear.
Cytokine production and autophagy
Autophagy is triggered by several environmental burdens, including cytokines. Moreover, autophagy can itself regulate the production of cytokines. The bi-directional interactions between cytokines and autophagy have been thoroughly reviewed.40 Herein, we will review some of the latest updates on the interplay of autophagy and cytokines secreted from and to DCs.
Regulation of cytokine production by autophagy
Autophagy was reported to be either positively or negatively involved in the production of pro- (but also anti-) inflammatory cytokines by DCs especially in the context of viral infection. Efficient viral immunity is attained by the massive production of the cytokine interferon alpha (IFN-α). This cytokine is massively secreted upon exposure of pDCs to the γ-herpes virus Epstein-Barr virus (EBV), human immunodeficiency virus type 1 (HIV-1), vesicular stomatitis virus (VSV) and paramyxovirus Simian Virus 5 SV5 by a mechanism requiring the autophagic machinery following TLR7/9 signaling.8,19,31,41 Notably, non-canonical autophagy has also been shown to regulate IFN-α production by pDCs upon TLR9 stimulation.42 Furthermore, BMDC infection with RSV was shown to induce the expression of the innate cytokines type I IFN, tumor necrosis factor (TNF), interleukin-6 (IL-6) and interleukin-12 (IL-12) p40 in an autophagy-dependent way, leading to subsequent T-cell activation.17 The CD4+ T-cell-mediated production of the cytokines IL-2 and IFN-γ upon parasitic infection with Toxoplasma gondii was shown to require ATG5 but not ATG7 in DCs.14 In addition to these pro-inflammatory cytokines, the production of the immunosuppressive cytokine IL-10 was also revealed to be inhibited by the blockade of autophagy either pharmacologically or by ATG16L1 knockdown in DCs generated from human peripheral blood mononuclear cells (PBMCs), leading to increased T-cell proliferation.20 By contrast, Tregs were shown to inhibit the autophagic machinery of DCs, leading to the improvement of autoimmune responses.33 Although contradictory, these data reveal a potential role of autophagy in DCs to control immune tolerance.
On the other hand, autophagy was also reported to inhibit interleukin-1β (IL-1β) production in BMDCs stimulated with LPS with ATP or alum by targeting pro-IL-1β for lysosomal degradation and by retaining the NLRP3 inflammasome activity.43 Haploinsufficiency of an essential autophagy protein, Beclin 1, which forms part of the class III PI3K complex involved in the early stages of autophagosome assembly, caused lower secretion of IL-6, TNF-α, IFN-β, IL-12p70 and IFN-γ in BMDCs.25 Furthermore, low autophagic activity and high release of IL-10, IL-6 and IL-23 were associated with DC exposure to Kaposi’s sarcoma-associated herpesvirus (KSHV).44 However, to the best of our knowledge, whether autophagy inhibition is responsible for the release of these cytokines was not explored.
Regulation of autophagy by cytokine production
We now know that many cytokines are potent inducers of autophagy in macrophages through studies on the role of autophagy in the macrophage response to Mycobacterium tuberculosis. However, very little is known about how this occurs in DCs. One of the best-characterized cytokines secreted by mature DCs is HMGB1. When secreted from mature DCs, this leaderless cytokine promotes T-cell and B-cell responses. When secreted from activated natural killer cells, it promotes DC maturation during the afferent immune response.45 Within the cytosol, HMGB1 promotes autophagy by releasing the essential autophagic gene Beclin 1 from its BCL-2 complex.46 However, whether HMGB1 regulates DC maturation by altering its autophagic levels remains to be elucidated.
In summary, a tight inter-regulation between cytokine production and autophagy in DCs allows them to perform their immune functions in both immunogenic and tolerogenic scenarios.
Antigen presentation and autophagy
The defining function of DCs as antigen-presenting cells is to internalize antigens and present antigen-derived peptides to T cells. A pioneering study showed that DCs exhibit a mild lysosomal proteolysis that retains antigens in lymphoid organs for extended periods and favors their presentation at the appropriate time.39 This observation highlights the peculiarity of DCs regarding the lysosomal degradation of their autophagic substrates that used to be exogenous antigens. DCs present endogenous and exogenous antigens to CD8+ T and CD4+ T cells through the formation of MHC class-I and class-II, respectively. Nevertheless, extracellular proteins can be presented by MHC-I by a process called cross-presentation. Strong evidence has highlighted the critical role of autophagy in MHC-II-mediated antigen presentation and, to a lesser extent, in MHC-I antigen presentation and cross-presentation. The antigen processing and presentation by autophagy in DCs has been thoroughly reviewed elsewhere;47 herein, we will summarize the latest developments in this topic with a special emphasis on canonical autophagy.
MHC-II-mediated antigen presentation and autophagy
MHC-II presentation of both self and non-self antigens was reported to require the autophagic machinery in DCs. Regarding self-antigens, autophagy was shown to be critical for MHC-II mediated presentation of citrullinated peptides to CD4+ T cells,48 highlighting the potential involvement of DC autophagy in autoimmunity. Regarding cancer and pathogenic antigens, tumor, viral and bacterial antigens were shown to be processed intracellularly for MHC-II presentation to CD4+ T cells via autophagy in DCs. For example, efficient presentation of the tumor antigen Mucin gene 1 on MHC-II by DCs requires lysosomal proteolysis through autophagy.49 The mycobacterial antigen Ag85B was also shown in vivo to require autophagy for its efficient presentation by MHC-II.24 Furthermore, autophagosome-targeted influenza matrix protein 1 led to their MHC-II presentation to CD4+ T cells50 and herpes simplex virus phagosomal antigens also need to be targeted to autophagosomes for CD4+ T-cell activation through MHC-II-mediated presentation.7 DCs infected with human immunodeficiency virus (HIV) and influenza virus were shown to not process MHC-II-restricted antigens independently of autophagy but to target their antigens to autophagosomes to favor MHC-II-restricted presentation and consequently trigger CD4+ T-cell responses.16,51 Conversely, a protein of Mycobacterium tuberculosis, PE_PGRS47, was shown to suppress autophagy and, consequently, MHC-II-restricted antigen presentation by mycobacteria-infected DCs, leading to its escape from host immune responses.52 Thus, the use of autophagy by infected DCs for or against the MHC-II restricted response may vary depending on the pathogen.
MHC-II presentation of extracellular (phagocytosed) antigens
Non-canonical autophagy plays an important role in the MHC-II-dependent presentation of phagocytosed antigens. The LAP pathway was shown to stabilize phagocytosed antigens for prolonged MHC-II antigen presentation.53 ATG5 deletion in DCs caused defects in the processing and MHC-II presentation of phagocytosed ovalbumin-coated beads,7 and LC3 deletion impaired the recruitment of MHC-II molecules to phagosomes and presentation of fungal-derived antigens to CD4 T cells.54
MHC-I-mediated antigen presentation and autophagy
Most of the peptides presented by MHC-I are derived from proteins processed by the ubiquitin-proteasome system (UPS) and then are transferred to the endoplasmic reticulum through the transporter associated with antigen processing (TAP). When autophagy is limited, some of its substrates, such as defective ribosomal products (DRiPs), accumulate in aggresome-like-induced structures (ALIS) and are subsequently presented by MHC-I after UPS-mediated processing.55 Through the modulation of DALIS formation, the co-chaperones— CHIP, BAG-1 and HspBP1—which regulate the interplay of Hsc70 and Hsp70 with UPS and autophagy, were shown to alter MHC-I-mediated antigen presentation in DCs.28 Autophagy was also shown to retain MHC-I internalization, leading to a lower stimulation of CD8+ T-cell responses.12 Recent reports have implicated a more direct role for autophagy in antigen presentation via MHC-I during viral infection through the non-classical TAP-independent presentation.56 However, the molecular mechanisms by which DCs by-pass the conventional MHC-I pathway to favor autophagic/lysosomal trafficking needs to be elucidated because it may be a key system to circumvent viral evasion that mainly targets the conventional UPS pathway. In this context, Herpes simplex virus 1 was recently suggested to impair DC ability to stimulate CD8 T cells by interfering with autophagic machinery through its viral protein ICP34.5.57
Cross-presentation and autophagy
The involvement of autophagy in the cross-presentation of antigens by DCs is controversial. Whereas the cross-presentation of viral peptides on MHC-I has been shown to remain intact after autophagy deletion in DCs,7 in other contexts, cross-presentation has been shown to be dependent on autophagy,33,58 including conjugated antigens to α-alumina nanoparticles in BMDCs,59 cells from patients with primary immunodeficiency chronic granulomatous disease (CGD) that lack phagocyte oxidase activity,60 and during yellow fever vaccination.13 Importantly, certain DC subsets seem to be particularly efficient at cross-presentation, namely, mouse CD8a-type DC (DC1) and their human counterpart CD1a+ DC.61 Villadangos and colleagues recently showed that the human CD1a+ subset and mouse DC1 subsets display high autophagic activity, which contributes to efficient cross-presentation of soluble antigens but not that of cell-associated antigen or antigen internalized via receptor-mediated endocytosis.18 However, the contribution of autophagy in this study was assessed by ATG7 deletion in CD11c+ DCs that comprise conventional DCs with and without cross-presentation activity. Hence, more specific deletion of ATG genes DCs specialized in cross-presentation is essential to discern the specific role of autophagy and exclude the possible intervention of other DC subsets in the regulation of this process. Importantly, a very recent study showed the contribution of autophagy in cross-presentation in murine CD8α+ DCs through VPS34, a subunit of the class III PI3K complex involved in autophagic trafficking.62 However, in this study, the VPS34 deletion was not specific to the CD8α+ DC subset.
Overall, DCs use the autophagic machinery to orchestrate T-cell responses by optimizing antigen processing and MHC-II presentation, as well as compromising MHC-I-restricted antigen presentation or enhancing some viral antigen presentation via MHC-I. Regarding the role of autophagy in antigen cross-presentation by DCs, it depends on the subset of DCs involved and nature of antigens exposed.
DC-mediated T-cell activation and autophagy
Autophagic activity in DCs is necessary for their ability to activate T cells. When stimulated by TLR4 and NOD2, BMDCs were shown to prime CD4+ T and CD8+ T cells in an autophagy-dependent way as judged by their ability to proliferate and secrete IFN-γ.38 Additionally, pharmacological inhibition of autophagy in BMDCs infected with RSV attenuated the production of the cytokines IL-17 and IFN-γ by CD4+ T cells.17 In agreement with this report, Beclin 1 was shown to be crucial for IFN-γ and IL-17 production by co-culturing CD4+ T cells and to negatively regulate the production of Th2 cytokines IL-5 and IL-13 during RSV infection.26 In line with the requirement of autophagy for DC-dependent CD4+ T-cell activation in viral infections, Beclin 1 haploinsufficiency in BMDCs infected with influenza A H1N1 virus decreased allogeneic CD4+ T-cell proliferation and induced CD4+ Foxp3+ regulatory T-cell differentiation, allowing H1N1 virus replication.25
Atg16L1-deficient mice demonstrate higher proliferation of CD4+, CD8+, and TCRγδ+ T cells due to increased DC numbers and costimulatory molecule expression.22 However, autophagy inhibition (by pharmacological inhibition or Atg16L1 knockdown) in DCs co-cultured with T cells and colonic epithelial cells resulted in lower IL-10 production by DCs, leading to significantly more T-cell proliferation.20 This finding suggests that autophagy in intestinal DCs may drive an opposite response in T-cell activation compared with that in other tissues. Importantly, during the development of the infectious disease Herpetic stromal keratitis, autophagy in DCs was shown to be an important pathway for CD4+ T-cell activation, subsequently leading to pathological corneal inflammation.15
Overall, several lines of experimental evidence have shown the requirement of autophagy in DC-mediated activation of T cells. This general observation is probably a consequence of the autophagy involvement in DC maturation as explained in the section above.
Activation of Tregs
Using ATG16L1-deficient murine BMDCs, non-canonical autophagy (ATG16L1, ATG5, and ATG7-dependent and Ulk1, Fip200, or Atg14-independent63) was found to be required to activate Tregs for the suppression of mucosal inflammation caused by the human commensal Bacteroides fragilis.23 Mechanistic studies have revealed that, for the protection of allograft rejection, the induction of tolerogenic DC generation that results in a higher production of CD25+ Foxp3+ Treg cells was associated with enhanced autophagy in the cardiac allograft.34 According to these two pioneering studies, autophagy may gain importance in the context of tolerance to drive the DC phenotype to tolerogenic functions. However, more mechanistic insights and in vivo experimental evidence are required to support this concept.
Conclusions and perspectives
In conclusion, several features of autophagy in DCs impact their immune functions within innate and especially adaptive immune response. Autophagy was recently shown to be indirectly or directly involved in the tolerogenic and immunogenic functions of DCs depending on the physiological context and micro-environment. Most of these studies rely on models with general deficiency of ATG genes. Given the complexity and divergence of DC subsets, targeting autophagic pathways in specific DC subsets will provide insight into the plasticity of DC functions. By taking advantage of autophagy inducers and inhibitors,64 the therapeutic correction of DC deregulation during infection and the anti-tumor immune response would be feasible.
One of the most important clinical contributions of DCs is their use in anti-cancer vaccination. Although they have been employed for nearly two decades in clinical trials for therapeutic vaccination against cancer, the tumor-induced tolerance renders those vaccinations ineffective with rates rarely exceeding 15%. Understanding how autophagy works in tolerogenic DCs in cancer will provide insights into potential strategies to overcome tumor-induced tolerance in DC vaccination approaches.
References
Knecht E, Aguado C, Cárcel J, Esteban I, Esteve JM, Ghislat G et al. Intracellular protein degradation in mammalian cells: recent developments. Cell Mol Life Sci [Internet] 2009; 66: 2427–2443. Available from http://www.ncbi.nlm.nih.gov/pubmed/19399586.
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A et al. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev Cell. 2011; 20: 131–139.
Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 2005; 22: 571–581.
Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M et al. Mammalian Autophagy: How Does It Work? Annu Rev Biochem [Internet] 2016; 85: 685–713. Available from http://www.annualreviews.org/doi/10.1146/annurev-biochem-060815-014556.
Romao S, Münz C. LC3-associated phagocytosis. Autophagy 2014; 10: 526–528.
Heckmann BL, Boada-Romero E, Cunha LD, Magne J, Green DR. LC3-Associated Phagocytosis and Inflammation. Vol. 429, Journal of Molecular Biology 2017; 429: 3561–3576.
Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A et al. In Vivo Requirement for Atg5 in Antigen Presentation by Dendritic Cells. Immunity 2010; 32: 227–239.
Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-Dependent Viral Recognition by Plasmacytoid Dendritic Cells. Science (80-) [Internet] 2007; 315: 1398–1401. Available from http://www.sciencemag.org/cgi/doi/10.1126/science.1136880.
Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 2010; 32: 654–669.
Weindel CG, Richey LJ, Mehta AJ, Shah M, Huber BT. Autophagy in Dendritic Cells and B Cells Is Critical for the Inflammatory State of TLR7-Mediated Autoimmunity. J Immunol [Internet] 2017; 198: 1081–1092. Available from http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1601307.
Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 2010; 16: 90–97.
Loi M, Müller A, Steinbach K, Niven J, Barreira da Silva R, Paul P et al. Macroautophagy Proteins Control MHC Class I Levels on Dendritic Cells and Shape Anti-viral CD8+ T Cell Responses. Cell Rep 2016; 15: 1076–1087.
Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS et al. Vaccine Activation of the Nutrient Sensor GCN2 in Dendritic Cells Enhances Antigen Presentation. Science (80-) [Internet] 2014; 343: 313–317. Available from http://www.sciencemag.org/cgi/doi/10.1126/science.1246829.
Liu E, Van Grol J, Subauste CS. Atg5 but not Atg7 in dendritic cells enhances IL-2 and IFN-gamma production by Toxoplasma gondii-reactive CD4+ T cells. Microbes Infect. 2015; 17: 275–284.
Jiang Y, Yin X, Stuart PM, Leib DA. Dendritic cell autophagy contributes to herpes simplex virus- driven stromal keratitis and immunopathology. MBio 2015; 6.
Coulon P-G, Richetta C, Rouers A, Blanchet FP, Urrutia A, Guerbois M et al. HIV-Infected Dendritic Cells Present Endogenous MHC Class II-Restricted Antigens to HIV-Specific CD4+T Cells. J Immunol [Internet] 2016; 197: 517–532. Available from http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1600286.
Morris S, Swanson MS, Lieberman A, Reed M, Yue Z, Lindell DM et al. Autophagy-Mediated Dendritic Cell Activation Is Essential for Innate Cytokine Production and APC Function with Respiratory Syncytial Virus Responses. J Immunol [Internet] 2011; 187: 3953–3961 Available from http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1100524.
Mintern JD, Macri C, Chin WJ, Panozza SE, Segura E, Patterson NL et al. Differential use of autophagy by primary dendritic cells specialized in cross-presentation. Autophagy 2015; 11: 906–917.
Zhou DJ, Kang KH, Spector SA. Production of Interferon alpha by Human Immunodeficiency Virus Type 1 in Human Plasmacytoid Dendritic Cells Is Dependent on Induction of Autophagy. J Infect Dis. 2012; 205: 1258–1267.
Strisciuglio C, Duijvestein M, Verhaar AP, Vos ACW, van den Brink GR, Hommes DW et al. Impaired autophagy leads to abnormal dendritic cell-epithelial cell interactions. J Crohn’s Colitis 2013; 7: 534–541.
Wildenberg ME, Koelink PJ, Diederen K, Te Velde AA, Wolfkamp SCS, Nuij VJ et al. The ATG16L1 risk allele associated with Crohn’s disease results in a Rac1-dependent defect in dendritic cell migration that is corrected by thiopurines. Mucosal Immunol 2017; 10: 352–360.
Hubbard-Lucey VM, Shono Y, Maurer K, West ML, Singer NV, Ziegler CGK et al. Autophagy Gene Atg16l1 Prevents Lethal T Cell Alloreactivity Mediated by Dendritic Cells. Immunity 2014; 41: 579–591.
Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science (80-) [Internet] 2016; 352: 1116–1120. Available from http://www.sciencemag.org/cgi/doi/10.1126/science.aad9948.
Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med 2009; 15: 267–276.
Zang F, Chen Y, Lin Z, Cai Z, Yu L, Xu F et al. Autophagy is involved in regulating the immune response of dendritic cells to influenza A (H1N1) pdm09 infection. Immunology 2016; 148: 56–69.
Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. J Immunol [Internet] 2013; 191: 2526–2537 Available from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3811020&tool=pmcentrez&rendertype=abstract.
Dalod M, Chelbi R, Malissen B, Lawrence T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. Vol. 33, EMBO Journal. 2014; 33: 1104–1116.
Kettern N, Rogon C, Limmer A, Schild H, Höhfeld J. The Hsc/Hsp70 co-chaperone network controls antigen aggregation and presentation during maturation of professional antigen presenting cells. PLoS One 2011; 6.
Min Y, Xu W, Liu D, Shen S, Lu Y, Zhang L et al. Autophagy promotes BCG-induced maturation of human dendritic cells. Acta Biochim Biophys Sin (Shanghai) 2010; 42: 177–182.
Luís A, Martins JD, Silva A, Ferreira I, Cruz MT, Neves BM. Oxidative stress-dependent activation of the eIF2α-ATFr unfolded protein response branch by skin sensitizer 1-fluoro-2,4-dinitrobenzene modulates dendritic-like cell maturation and inflammatory status in a biphasic manner. Free Radic Biol Med. 2014; 77: 217–229.
Manuse MJ, Briggs CM, Parks GD. Replication-independent activation of human plasmacytoid dendritic cells by the paramyxovirus SV5 Requires TLR7 and autophagy pathways. Virology 2010; 405: 383–389.
Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S et al. TIM-4 Glycoprotein-Mediated Degradation of Dying Tumor Cells by Autophagy Leads to Reduced Antigen Presentation and Increased Immune Tolerance. Immunity 2013; 39: 1070–1081.
Alissafi T, Banos A, Boon L, Sparwasser T, Ghigo A, Wing K et al. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J Clin Invest. 2017; 127: 2789–2804.
Xiong A, Duan L, Chen J, Fan Z, Zheng F, Tan Z et al. Flt3L Combined with Rapamycin Promotes Cardiac Allograft Tolerance by Inducing Regulatory Dendritic Cells and Allograft Autophagy in Mice. PLoS One 2012; 7.
Ghislat G, Knecht E. Ca2+-sensor proteins in the autophagic and endocytic traffic. Curr Protein Pept Sci [Internet] 2013; 14: 97–110. Available from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3664516&tool=pmcentrez&rendertype=abstract.
Xu Y, Jagannath C, De Liu X, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like Receptor 4 Is a Sensor for Autophagy Associated with Innate Immunity. Immunity 2007; 27: 135–144.
Terawaki S, Camosseto V, Prete F, Wenger T, Papadopoulos A, Rondeau C et al. RUN and FYVE domain-containing protein 4 enhances autophagy and lysosome tethering in response to Interleukin-4. J Cell Biol. 2015; 210: 1133–1152.
Khan N, Vidyarthi A, Pahari S, Negi S, Aqdas M, Nadeem S et al. Signaling through NOD-2 and TLR-4 Bolsters the T cell Priming Capability of Dendritic cells by Inducing Autophagy. Sci Rep 2016; 6.
Delamarre L. Differential Lysosomal Proteolysis in Antigen-Presenting Cells Determines Antigen Fate. Science (80-) [Internet] 2005; 307: 1630–1634. Available from http://www.sciencemag.org/cgi/doi/10.1126/science.1108003.
Harris J. Autophagy and cytokines. Vol. 56, Cytokine 2011; 56: 140–144.
Severa M, Giacomini E, Gafa V, Anastasiadou E, Rizzo F, Corazzari M et al. EBV stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: Implications for viral immune escape. Eur J Immunol. 2013; 43: 147–158.
Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L et al. Noncanonical Autophagy Is Required for Type I Interferon Secretion in Response to DNA-Immune Complexes. Immunity 2012; 37: 986–997.
Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem [Internet] 2011; 286: 9587–9597 Available from http://www.ncbi.nlm.nih.gov/pubmed/21228274.
Santarelli R, Gonnella R, Di Giovenale G, Cuomo L, Capobianchi A, Granato M et al. STAT3 activation by KSHV correlates with IL-10, IL-6 and IL-23 release and an autophagic block in dendritic cells. Sci Rep 2014; 4.
Li G, Liang X, Lotze MT. HMGB1: The central cytokine for all lymphoid cells. Vol. 4, Frontiers in Immunology 2013; 4.
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010; 190: 881–892.
Münz C. Autophagy proteins in antigen processing for presentation on MHC molecules. Vol. 272, Immunological Reviews. 2016; 272: 17–27.
Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med [Internet] 2011; 208: 2625–2632. Available from http://www.jem.org/lookup/doi/10.1084/jem.20110640.
Dörfel D, Appel S, Grünebach F, Weck MM, Müller MR, Heine A et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood 2005; 105: 3199–3205.
Schmid D, Pypaert M, Münz C. Antigen-Loading Compartments for Major Histocompatibility Complex Class II Molecules Continuously Receive Input from Autophagosomes. Immunity 2007; 26: 79–92.
Comber JD, Robinson TM, Siciliano NA, Snook AE, Eisenlohr LC. Functional Macroautophagy Induction by Influenza A Virus without a Contribution to Major Histocompatibility Complex Class II-Restricted Presentation. J Virol [Internet] 2011; 85: 6453–6463. Available from http://jvi.asm.org/cgi/doi/10.1128/JVI.02122-10.
Saini NK, Baena A, Ng TW, Venkataswamy MM, Kennedy SC, Kunnath-Velayudhan S et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE-PGRS47. Nat Microbiol 2016; 1.
Romao S, Gasser N, Becker AC, Guhl B, Bajagic M, Vanoaica D et al. Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing. J Cell Biol. 2013; 203: 757–766.
Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012; 287: 34149–34156.
Wenger T, Terawaki S, Camosseto V, Abdelrassoul R, Mies A, Catalan N et al. Autophagy inhibition promotes defective neosynthesized proteins storage in ALIS, and induces redirection toward proteasome processing and MHCI-restricted presentation. Autophagy 2012; 8: 350–363.
Tey SK, Khanna R. Autophagy mediates transporter associated with antigen processing- independent presentation of viral epitopes through MHC class I pathway. Blood 2012; 120: 994–1004.
Budida R, Stankov MV, Döhner K, Buch A, Panayotova-Dimitrova D, Tappe KA et al. Herpes simplex virus 1 interferes with autophagy of murine dendritic cells and impairs their ability to stimulate CD8+T lymphocytes. Eur J Immunol [Internet] 2017; 47: 1819–1834 Available from http://doi.wiley.com/10.1002/eji.201646908.
Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009; 16: 991–1005.
Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol 2011; 6: 645–650.
De Luca A, Iannitti RG, Bozza S, Beau R, Casagrande A, D’Angelo C et al. CD4+T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease. J Clin Invest. 2012; 122: 1816–1831.
Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: One general or all sergeants? Vol. 34, Trends in Immunology. 2013; 34: 361–370.
Parekh VV, Pabbisetty SK, Wu L, Sebzda E, Martinez J, Zhang J et al. Autophagy-related protein Vps34 controls the homeostasis and function of antigen cross-presenting CD8α+dendritic cells. Proc Natl Acad Sci [Internet] 2017, 201706504 Available from http://www.pnas.org/lookup/doi/10.1073/pnas.1706504114.
Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 2015; 17: 893–906.
Kroemer G. Autophagy: A druggable process that is deregulated in aging and human disease. Vol. 125, Journal of Clinical Investigation 2015; 125: 1–4.
Acknowledgements
The present work was supported by funding from the Horizon 2020 work program of the Marie Curie Actions grant agreement number 57212 MCNTMTDC (GG) and La Ligue Contre le Cancer fellowship (GG) and Equipe Labellisee LNCC grant (TL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Ghislat, G., Lawrence, T. Autophagy in dendritic cells. Cell Mol Immunol 15, 944–952 (2018). https://doi.org/10.1038/cmi.2018.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2018.2
Keywords
This article is cited by
-
TFEB and TFE3 cooperate in regulating inorganic arsenic-induced autophagy-lysosome impairment and immuno-dysfunction in primary dendritic cells
Cell Biology and Toxicology (2024)
-
Function and autophagy of monocyte-derived dendritic cells is affected by hepatitis B virus infection
BMC Immunology (2023)
-
MHBSt167 induced autophagy promote cell proliferation and EMT by activating the immune response in L02 cells
Virology Journal (2022)
-
Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming
Mucosal Immunology (2022)
-
TNF-α-induced protein 8-like 2 negatively regulates the immune function of dendritic cells by suppressing autophagy via the TAK1/JNK pathway in septic mice
Cell Death & Disease (2021)