Abstract
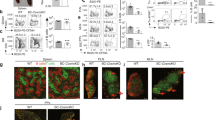
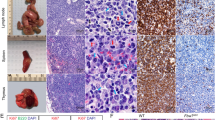
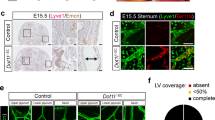
B cells home to the lymph nodes (LNs) via high endothelial venules (HEVs) under the guidance of chemokines, particularly CXCL13. However, as CXCL13 is not directly made in HEVs, the molecular mechanism mediating B-cell homing to LNs has remained unclear. We show here that nuclear factor (NF)-κB-inducing kinase (NIK), a kinase mediating activation of the noncanonical NF-κB pathway, functions in lymphatic endothelial cells (LECs) to regulate B-cell homing to LNs. LEC-conditional deletion of NIK in mice did not affect the integrity or global function of lymphatic vessels but caused a severe reduction in the frequency of B cells in LNs. The LEC-specific NIK deficiency did not affect the survival of B cells or the frequency of B cells in the spleen. B-cell adoptive transfer studies revealed that the LEC-specific NIK deletion impairs the ability of LNs to recruit B cells. We further show that NIK mediates expression of the chemokines CXCL13 and CCL19 in LECs. Although CCL19 is also expressed in blood endothelial cells (BECs), CXCL13 is not produced in BECs. These results suggest that NIK regulates naive B-cell homing to LNs via mediating production of the B-cell homing chemokine CXCL13 in LECs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol 1959; 146: 54–69.
Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995; 16: 449–457.
Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004; 4: 360–370.
Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012; 12: 762–773.
von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003; 3: 867–878.
Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996; 272: 60–66.
Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33.
Nakano H, Tamura T, Yoshimoto T, Yagita H, Miyasaka M, Butcher EC et al. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur J Immunol 1997; 27: 215–221.
Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med 2001; 193: 1105–1112.
Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol 2003; 171: 1642–1646.
Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein—coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 1994; 84: 830–840.
Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999; 144: 789–801.
Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol 2008; 3: 367–397.
Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS 2004; 112: 526–538.
Kesler CT, Liao S, Munn LL, Padera TP. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med 2013; 5: 111–124.
Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev 2010; 24: 2115–2126.
Yeo KP, Angeli V. Bidirectional crosstalk between lymphatic endothelial cell and T cell and its implications in tumor immunity. Front Immunol 2017; 8: 83.
Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 2017; 17: 545–558.
Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev 2012; 246: 125–140.
Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 2017; 17: 545–558.
van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol 2010; 10: 664–674.
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001; 293: 1495–1499.
Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 2001; 7: 401–409.
Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet 1999; 22: 74–77.
Brightbill HD, Jackman JK, Suto E, Kennedy H, Jones C 3rd, Chalasani S et al. Conditional deletion of NF-kappaB-inducing kinase (NIK) in adult mice disrupts mature B cell survival and activation. J Immunol 2015; 195: 953–964.
Maijer KI, Noort AR, de Hair MJ, van der Leij C, van Zoest KP, Choi IY et al. Nuclear factor-kappaB-inducing kinase is expressed in synovial endothelial cells in patients with early arthritis and correlates with markers of inflammation: a prospective cohort study. J Rheumatol 2015; 42: 1573–1581.
Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 2010; 207: 17–27.
Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479: 542–546.
Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006; 25: 153–162.
Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 2016; 1: e85096.
Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 2007; 8: 1255–1265.
Thomas MD, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell Immunol 2006; 239: 92–102.
Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol 2011; 2: 35.
Martin-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol 2009, 31–49.
Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 2008; 8: 362–371.
Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol 2000; 10: R30–R33.
Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol 2012; 13: 681–690.
Saeki H, Wu MT, Olasz E, Hwang ST. A migratory population of skin-derived dendritic cells expresses CXCR5, responds to B lymphocyte chemoattractant in vitro, and co-localizes to B cell zones in lymph nodes in vivo. Eur J Immunol 2000; 30: 2808–2814.
Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH et al. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol 2002; 168: 5117–5123.
Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 2004; 21: 279–288.
De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol 2015; 15: 137–148.
Yamada T, Mitani T, Yorita K, Uchida D, Matsushima A, Iwamasa K et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol 2000; 165: 804–812.
Shinkura R, Matsuda F, Sakiyama T, Tsubata T, Hiai H, Paumen M et al. Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice. Int Immunol 1996; 8: 1067–1075.
Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol 1994; 24: 429–434.
Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel RM. Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol 2000; 30: 2799–2807.
Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004; 22: 129–156.
Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol 2009; 9: 618–629.
Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998; 95: 258–263.
Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 2000; 97: 12694–12699.
Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 1998; 391: 799–803.
Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006; 25: 989–1001.
Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 2002; 17: 525–535.
Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 2005; 23: 787–819.
Kumar V, Dasoveanu DC, Chyou S, Tzeng TC, Rozo C, Liang Y et al. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity 2015; 42: 719–730.
Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K et al. In vivo analysis of dendritic cell development and homeostasis. Science 2009; 324: 392–397.
Seth S, Oberdorfer L, Hyde R, Hoff K, Thies V, Worbs T et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol 2011; 186: 3364–3372.
Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 2005; 22: 493–505.
Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999; 189: 451–460.
Acknowledgements
We thank Genentech Inc. for providing the NIK-flox mice. This work was supported by grants from the National Institutes of Health (GM84459, AI057555, AI104519 and AI64639). This study also used the NIH/NCI-supported resources under award number P30CA016672 at The MD Anderson Cancer Center. SZ was supported by a scholarship from the China Scholarship Council (CSC) under the Grant CSC 201506210393. We also thank Professor Wei He for his support.
Author information
Authors and Affiliations
Contributions
JY and SZ designed and performed the experiments, and JY prepared the figures and wrote the manuscript. LZ, XX, HW, ZJ, MG, J-YY and XC contributed to the performance of the experiments. S-CS supervised the work and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, J., Zhang, S., Zhang, L. et al. Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism. Cell Mol Immunol 16, 165–177 (2019). https://doi.org/10.1038/cmi.2017.167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2017.167