Abstract
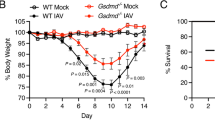
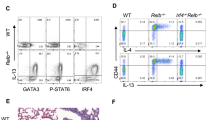
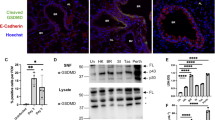
The ubiquitin ligase, Itch, is required to prevent autoinflammatory disease in mice and humans. Itch-deficient mice develop lethal pulmonary inflammation characterized by the production of Th2 cytokines (for example, interleukin-4 (IL-4)); however, the contribution of Itch to immune defense against respiratory pathogens has not been determined. We found that Itch-deficient mice were highly susceptible to intranasal infection with the respiratory pathogen Klebsiella pneumoniae. Infected Itch-deficient mice exhibited increased immune cell infiltration, cytokine levels and bacterial burden in the respiratory tract compared with control mice. However, numbers of resident alveolar macrophages were reduced in the lungs from Itch-deficient mice both before and after infection. High levels of Th2 cytokines in the respiratory tract correlated with deceased alveolar macrophages, and genetic ablation of IL-4 restored alveolar macrophages and host defense to K. pneumoniae in Itch-deficient mice, suggesting that loss of alveolar macrophages occurred as a consequence of Th2 inflammation. Adoptive transfer of Itch−/− CD4+ T cells into Rag−/− mice was sufficient to drive reduction in numbers of Itch-replete alveolar macrophages. Finally, we found that Stat6 signaling downstream of the IL-4 receptor directly reduced fitness of alveolar macrophages when these cells were exposed to the Itch−/− inflamed respiratory tract. These data suggest that Th2 inflammation directly impairs alveolar macrophage fitness in Itch−/− mice, and elucidate a previously unappreciated link between Th2 cells, alveolar macrophages and susceptibility to bacterial infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 2001; 70: 503–533.
Perry WL, Hustad CM, Swing DA, O'Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet 1998; 18: 143–146.
Lohr NJ, Molleston JP, Strauss KA, Torres-Martinez W, Sherman EA, Squires RH et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet 2010; 86: 447–453.
Santini S, Stagni V, Giambruno R, Fianco G, Di Benedetto A, Mottolese M et al. ATM kinase activity modulates ITCH E3-ubiquitin ligase activity. Oncogene 2014; 33: 1113–1123.
Schroeder SA, Zielen S. Infections of the respiratory system in patients with ataxia-telangiectasia. Pediatr Pulmonol 2014; 49: 389–399.
Hustad CM, Perry WL, Siracusa LD, Rasberry C, Cobb L, Cattanach BM et al. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics 1995; 140: 255–265.
Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol 2002; 3: 281–287.
Parravicini V, Field AC, Tomlinson PD, Basson MA, Zamoyska R. Itch-/- alphabeta and gammadelta T cells independently contribute to autoimmunity in Itchy mice. Blood 2008; 111: 4273–7282.
Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol 2008; 9: 254–262.
Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T et al. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol 2008; 9: 245–253.
Ahmed N, Zeng M, Sinha I, Polin L, Wei WZ, Rathinam C et al. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat Immunol 2011; 12: 1176–1183.
Enzler T, Chang X, Facchinetti V, Melino G, Karin M, Su B et al. MEKK1 binds HECT E3 ligase Itch by its amino-terminal RING motif to regulate Th2 cytokine gene expression. J Immunol 2009; 183: 3831–3838.
Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370: 1198–1208.
Berendt RF. Relationship of method of administration to respiratory virulence of Klebsiella pneumoniae for mice and squirrel monkeys. Infect Immun 1978; 20: 581–583.
Lawlor MS, Hsu J, Rick PD, Miller VL. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 2005; 58: 1054–1073.
Xiong H, Carter RA, Leiner IM, Tang YW, Chen L, Kreiswirth BN et al. Distinct Contributions of Neutrophils and CCR2+ Monocytes to Pulmonary Clearance of Different Klebsiella pneumoniae Strains. Infect Immun 2015; 83: 3418–3427.
Kostina E, Ofek I, Crouch E, Friedman R, Sirota L, Klinger G et al. Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D. Infect Immun 2005; 73: 8282–8290.
Steichen AL, Binstock BJ, Mishra BB, Sharma J. C-type lectin receptor Clec4d plays a protective role in resolution of Gram-negative pneumonia. J Leukoc Biol 2013; 94: 393–398.
Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev 2000; 173: 39–51.
Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R 3rd et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 1997; 65: 1139–1146.
Divangahi M, King IL, Pernet E. Alveolar macrophages and type I IFN in airway homeostasis and immunity. Trends Immunol 2015; 36: 307–314.
Holt PG. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol 1986; 63: 261–270.
Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004; 31: 3–7.
Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 2013; 31: 317–343.
Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann NY Acad Sci 1993; 685: 713–739.
Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood 2008; 112: 111–119.
Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun 2005; 73: 532–545.
Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014; 506: 503–506.
Ramon HE, Riling CR, Bradfield J, Yang B, Hakonarson H, Oliver PM. The ubiquitin ligase adaptor Ndfip1 regulates T cell-mediated gastrointestinal inflammation and inflammatory bowel disease susceptibility. Mucosal Immunol 2011; 4: 314–324.
Tian F, Han Y, Song J, Lei J, Yan X, Xie N et al. Pulmonary resident neutrophils regulate the production of GM-CSF and alveolar macrophages. FEBS J 2016; 283: 1465–1474.
Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Ann Rev Physiol 2002; 64: 775–802.
Zhang M, Veselits M, O'Neill S, Hou P, Reddi AL, Berlin I et al. Ubiquitinylation of Ig beta dictates the endocytic fate of the B cell antigen receptor. J Immunol 2007; 179: 4435–4443.
Tao M, Scacheri PC, Marinis JM, Harhaj EW, Matesic LE, Abbott DW. ITCH K63-ubiquitinates the NOD2 binding protein, RIP2, to influence inflammatory signaling pathways. Curr Biol 2009; 19: 1255–1263.
Zhang H, Wu C, Matesic LE, Li X, Wang Z, Boyce BF et al. Ubiquitin E3 ligase Itch negatively regulates osteoclast formation by promoting deubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem 2013; 288: 22359–22368.
Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol 2014; 15: 657–666.
Chen WH, Toapanta FR, Shirey KA, Zhang L, Giannelou A, Page C et al. Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza. Immunol Lett 2012; 141: 227–234.
Song Z, Zhang J, Zhang X, Li D, Wang H, Xu X et al. Interleukin 4 deficiency reverses development of secondary pseudomonas aeruginosa pneumonia during sepsis-associated immunosuppression. J Infect Dis 2015; 211: 1616–1627.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol 2010; 135: 223–235.
Dagvadorj J, Shimada K, Chen S, Jones HD, Tumurkhuu G, Zhang W et al. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin-1alpha release. Immunity 2015; 42: 640–653.
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L et al. TLR4-upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep 2016; 6: 31663.
Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Ann Rev Immunol 2013; 31: 317–343.
Siracusa MC, Reece JJ, Urban Jr JF, Scott AL. Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol 2008; 84: 1422–1433.
Benoit M, Desnues B, Mege J-L. Macrophage polarization in bacterial infections. J Immunol 2008; 181: 3733–3739.
Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A et al. M2 Macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol 2009; 182: 4415–4422.
Marino A, Menghini R, Fabrizi M, Casagrande V, Mavilio M, Stoehr R et al. ITCH deficiency protects from diet-induced obesity. Diabetes 2014; 63: 550–561.
Acknowledgements
We give special thanks to Junjie Mei in the Worthen lab for assistance with the K. pneumoniae infection model, as well as to Theresa Leichner in the Kimbayashi lab for providing Stat6−/− mice. In addition, we thank Stephanie Sprout for outstanding technical help. This research was funded by the following sources: The National Institutes of Health, (R01AI093566 and R01AI114515) and the American Asthma Foundation (AAF 13-0020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moser, E.K., Field, N.S. & Oliver, P.M. Aberrant Th2 inflammation drives dysfunction of alveolar macrophages and susceptibility to bacterial pneumonia. Cell Mol Immunol 15, 480–492 (2018). https://doi.org/10.1038/cmi.2016.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.69
This article is cited by
-
The ubiquitin ligase Cul5 regulates CD4+ T cell fate choice and allergic inflammation
Nature Communications (2022)
-
Role of sleep deprivation in immune-related disease risk and outcomes
Communications Biology (2021)