Abstract
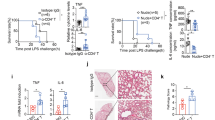
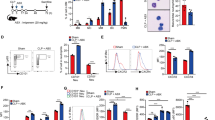
Cold-inducible RNA-binding protein (CIRP) is a novel inflammatory mediator that stimulates the release of proinflammatory cytokines from macrophages in sepsis. Given the immune dysregulation that characterizes sepsis, the effect of CIRP on other immune cells is an area of increasing interest that has not yet been studied. In the present study, we hypothesized that extracellular CIRP promotes activation of T lymphocytes in the spleen during sepsis. We observed that mice subjected to sepsis by cecal ligation and puncture showed significantly higher expression of the early activation markers CD69 and CD25 at 20 h on CD4+ splenic T cells, and significantly higher CD69 expression on CD8+ splenic T cells compared with sham-operated controls. Furthermore, at 20 h after receiving intravenous injection of recombinant murine CIRP (rmCIRP, 5 mg/kg body weight (BW)) or PBS (vehicle), those mice receiving rmCIRP showed significantly increased expression of CD69 and CD25 on both CD4+ and CD8+ splenic T cells. This effect, however, was not seen in TLR4-deficient mice after rmCIRP injection. In addition, treatment with CIRP predisposed CD4+ T cells to a Th1 hyperinflammatory response profile, and influenced CD8+ T cells toward a cytotoxic profile. Taken together, our findings indicate that CIRP is a proinflammatory mediator that plays an important role in T-cell dysregulation during sepsis in a TLR4-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810.
Angus DC, van der Poll T . Severe sepsis and septic shock. N Engl J Med 2013; 369: 2063.
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193: 259–272.
Scumpia PO, Delano MJ, Kelly KM, O'Malley KA, Efron PA, McAuliffe PF et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006; 177: 7943–7949.
Martin GS . Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 2012; 10: 701–706.
Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40: 754–761.
Aziz M, Jacob A, Yang WL, Matsuda A, Wang P . Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 2013; 93: 329–342.
Hotchkiss RS, Monneret G, Payen D . Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013; 13: 260–268.
Chen GY, Nunez G . Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837.
Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 2013; 19: 1489–1495.
Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J . A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 1997; 137: 899–908.
Morf J, Rey G, Schneider K, Stratmann M, Fujita J, Naef F et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 2012; 338: 379–383.
Zhu X, Bührer C, Wellmann S . Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci 2016 e-pub ahead of print 4 May 2016 doi:10.1007/s00018-016-2253-7.
González-Amaro R, Cortés JR, Sánchez-Madrid F, Martín P . Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med 2013; 19: 625–632.
Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, Fiorentini S et al. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 1997; 27: 71–76.
van Schaik SM, Abbas AK . Role of T cells in a murine model of Escherichia coli sepsis. Eur J Immunol 2007; 37: 3101–3110.
Roger PM, Hyvernat H, Ticchioni M, Kumar G, Dellamonica J, Bernardin G . The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J Crit Care 2012; 27: 384–393.
Guo L, Liu F, Lu MP, Zheng Q, Chen ZM . Increased T cell activation in BALF from children with Mycoplasma pneumoniae pneumonia. Pediatr Pulmonol 2015; 50: 814–819.
Schmoeckel K, Traffehn S, Eger C, Potschke C, Broker BM . Full activation of CD4+ T cells early during sepsis requires specific antigen. Shock 2015; 43: 192–200.
Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A . The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock 2007; 27: 251–257.
Zhou Y, Dong H, Zhong Y, Huang J, Lv J, Li J . The cold-inducible RNA–binding protein (CIRP) level in peripheral blood predicts sepsis outcome. PLoS One 2015; 10: e0137721.
Cesta MF . Normal structure, function, and histology of the spleen. Toxicol Pathol 2006; 34: 455–465.
The Jackson Laboratory Flow-Cytometric Analysis of 11 Strains of Mice. MPD:Jaxpheno6. Mouse Phenome Database Website. The Jackson Laboratory: Bar Harbor, Maine: USA. Available at: http://phenome.jax.org. (accessed on 6 March 2016).
Radulovic K, Niess JH . CD69 is the crucial regulator of intestinal inflammation: a new target molecule for IBD treatment? J Immunol Res 2015; 2015: 497056.
Martin P, Gomez M, Lamana A, Matesanz Marin A, Cortes JR, Ramirez-Huesca M et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J Allergy Clin Immunol 2010; 126: 355–365; 65.e1-3.
Sancho D, Gomez M, Viedma F, Esplugues E, Gordon-Alonso M, Garcia-Lopez MA et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest 2003; 112: 872–882.
Radulovic K, Manta C, Rossini V, Holzmann K, Kestler HA, Wegenka UM et al. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J Immunol 2012; 188: 2001–2013.
Sharma A, Yang WL, Matsuo S, Wang P . Differential alterations of tissue T-cell subsets after sepsis. Immunol Lett 2015; 168: 41–50.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999; 285: 248–251.
Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 2003; 284: C870–C879.
Zhang LT, Yao YM, Dong YQ, Dong N, Yu Y, Sheng ZY . Relationship between high-mobility group box 1 protein release and T-cell suppression in rats after thermal injury. Shock 2008; 30: 449–455.
Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999; 27: 1230–1251.
Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA . Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 2005; 174: 3765–3772.
Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 2001; 166: 6952–6963.
Cao C, Ma T, Chai YF, Shou ST . The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med 2015; 6: 5–9.
Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A et al. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit Care Med 2004; 32: 2329–2331.
Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F et al. Increased circulating regulatory T cells (CD4+CD25+CD127−) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med 2009; 35: 678–686.
Han Y, Guo Q, Zhang M, Chen Z, Cao X . CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol 2009; 182: 111–120.
Zhao XS, Wang XH, Zhao XY, Chang YJ, Xu LP, Zhang XH et al. Non-traditional CD4+CD25-CD69+ regulatory T cells are correlated to leukemia relapse after allogeneic hematopoietic stem cell transplantation. J Transl Med 2014; 12: 187.
Mukherjee S, Karmakar S, Babu SP . TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis 2016; 20: 193–204.
Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol 2007; 179: 41–44.
Zanin-Zhorov A, Cohen IR . Signaling via TLR2 and TLR4 directly down-regulates T cell effector functions: the regulatory face of danger signals. Front Immunol 2013; 4: 211.
Reynolds JM, Martinez GJ, Chung Y, Dong C . Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA 2012; 109: 13064–13069.
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH . A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000; 100: 655–669.
Schoenborn JR, Wilson CB . Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96: 41–101.
Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ . T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 2012; 37: 501–510.
Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol 2005; 77: 914–922.
Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R . Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2001; 2: 947–950.
Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ . From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother 2005; 49: 3991–3996.
Voskoboinik I, Whisstock JC, Trapani JA . Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015; 15: 388–400.
Appay V, Rowland-Jones SL . RANTES: a versatile and controversial chemokine. Trends Immunol 2001; 22: 83–87.
Acknowledgements
We thank Dr Kevin Tracey for providing the Tlr4−/− mice and the members of the Flow Cytometry Core Facility at the Feinstein Institute for Medical Research for their input and technical assistance. This study was supported by the National Institutes of Health (NIH) Grants HL076179 and GM053008 (PW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website
Supplementary information
Rights and permissions
About this article
Cite this article
Bolognese, A., Sharma, A., Yang, WL. et al. Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner. Cell Mol Immunol 15, 38–47 (2018). https://doi.org/10.1038/cmi.2016.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.43
Keywords
This article is cited by
-
Extracellular CIRP induces CD4CD8αα intraepithelial lymphocyte cytotoxicity in sepsis
Molecular Medicine (2024)
-
Cold-inducible RNA binding protein alleviates iron overload-induced neural ferroptosis under perinatal hypoxia insult
Cell Death & Differentiation (2024)
-
Activation of immune signals during organ transplantation
Signal Transduction and Targeted Therapy (2023)
-
Evaluation of serum and tissue levels of cold-inducible RNA-binding protein in non-segmental Vitiligo
Archives of Dermatological Research (2023)
-
Extracellular CIRP dysregulates macrophage bacterial phagocytosis in sepsis
Cellular & Molecular Immunology (2022)