Abstract
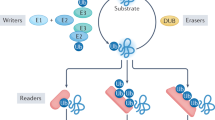
The ubiquitin system comprises enzymes that are responsible for ubiquitination and deubiquitination, as well as ubiquitin receptors that are capable of recognizing and deciphering the ubiquitin code, which act in coordination to regulate almost all host cellular processes, including host–pathogen interactions. In response to pathogen infection, the host innate immune system launches an array of distinct antimicrobial activities encompassing inflammatory signaling, phagosomal maturation, autophagy and apoptosis, all of which are fine-tuned by the ubiquitin system to eradicate the invading pathogens and to reduce concomitant host damage. By contrast, pathogens have evolved a cohort of exquisite strategies to evade host innate immunity by usurping the ubiquitin system for their own benefits. Here, we present recent advances regarding the ubiquitin system-mediated modulation of host–pathogen interplay, with a specific focus on host innate immune defenses and bacterial pathogen immune evasion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kerscher O, Felberbaum R, Hochstrasser M . Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 2006; 22: 159–180.
Komander D, Rape M . The ubiquitin code. Annu Rev Biochem 2012; 81: 203–229.
Deshaies RJ, Joazeiro CA . RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009; 78: 399–434.
Rotin D, Kumar S . Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 2009; 10: 398–409.
Smit JJ, Sixma TK . RBR E3-ligases at work. EMBO Rep 2014; 15: 142–154.
Smit JJ, Monteferrario D, Noordermeer SM, van Dijk WJ, van der Reijden BA, Sixma TK . The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. EMBO J 2012; 31: 3833–3844.
Chen ZJ, Sun LJ . Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 2009; 33: 275–286.
Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 2009; 137: 133–145.
Meyer HJ, Rape M . Enhanced protein degradation by branched ubiquitin chains. Cell 2014; 157: 910–921.
Husnjak K, Dikic I . Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem 2012; 81: 291–322.
Komander D, Clague MJ, Urbe S . Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 2009; 10: 550–563.
Jiang X, Chen ZJ . The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol 2012; 12: 35–48.
Takeuchi O, Akira S . Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820.
Zinngrebe J, Montinaro A, Peltzer N, Walczak H . Ubiquitin in the immune system. EMBO Rep 2014; 15: 28–45.
Caruso R, Warner N, Inohara N, Nunez G . NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 2014; 41: 898–908.
Bertrand MJ, Doiron K, Labbe K, Korneluk RG, Barker PA, Saleh M . Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 2009; 30: 789–801.
Tao M, Scacheri PC, Marinis JM, Harhaj EW, Matesic LE, Abbott DW . ITCH K63-ubiquitinates the NOD2 binding protein, RIP2, to influence inflammatory signaling pathways. Curr Biol 2009; 19: 1255–1263.
Abbott DW, Yang Y, Hutti JE, Madhavarapu S, Kelliher MA, Cantley LC . Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol Cell Biol 2007; 27: 6012–6025.
Ver Heul AM, Fowler CA, Ramaswamy S, Piper RC . Ubiquitin regulates caspase recruitment domain-mediated signaling by nucleotide-binding oligomerization domain-containing proteins NOD1 and NOD2. J Biol Chem 2013; 288: 6890–6902.
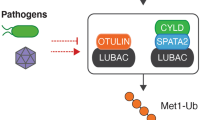
Damgaard RB, Nachbur U, Yabal M, Wong WW, Fiil BK, Kastirr M et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell 2012; 46: 746–758.
Fiil BK, Damgaard RB, Wagner SA, Keusekotten K, Fritsch M, Bekker-Jensen S et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol Cell 2013; 50: 818–830.
Hrdinka M, Fiil BK, Zucca M, Leske D, Bagola K, Yabal M et al. CYLD limits Lys63- and Met1-linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Rep 2016; 14: 2846–2858.
Jorgensen I, Miao EA . Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015; 265: 130–142.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665.
Franchi L, Munoz-Planillo R, Nunez G . Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13: 325–332.
Bednash JS, Mallampalli RK . Regulation of inflammasomes by ubiquitination. Cell Mol Immunol 2016; 13: 1–7.
Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009; 113: 2324–2335.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D et al. Cutting edge: NF- B activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183: 787–791.
Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J Biol Chem 2015; 290: 18124–18133.
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J . Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 2013; 49: 331–338.
Rodgers MA, Bowman JW, Fujita H, Orazio N, Shi M, Liang Q et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med 2014; 211: 1333–1347.
Labbe K, McIntire CR, Doiron K, Leblanc PM, Saleh M . Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 2011; 35: 897–907.
Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA et al. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity 2015; 42: 55–67.
Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 2012; 13: 255–263.
Fairn GD, Grinstein S . How nascent phagosomes mature to become phagolysosomes. Trends Immunol 2012; 33: 397–405.
Stenmark H . Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10: 513–525.
Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M . Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 2001; 12: 2219–2228.
Mattera R, Bonifacino JS . Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes. EMBO J 2008; 27: 2484–2494.
Lee S, Tsai YC, Mattera R, Smith WJ, Kostelansky MS, Weissman AM et al. Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5. Nat Struct Mol Biol 2006; 13: 264–271.
Clague MJ, Urbe S . Endocytosis: the DUB version. Trends Cell Biol 2006; 16: 551–559.
Song P, Trajkovic K, Tsunemi T, Krainc D . Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J Neurosci 2016; 36: 2425–2437.
Piper RC, Katzmann DJ . Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 2007; 23: 519–547.
Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog 2013; 9: e1003734.
Henne WM, Buchkovich NJ, Emr SD . The ESCRT pathway. Dev Cell 2011; 21: 77–91.
Raiborg C, Stenmark H . The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009; 458: 445–452.
Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol 2006; 8: 163–169.
Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L . Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol Biol Cell 2007; 18: 1129–1142.
Sierra MI, Wright MH, Nash PD . AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4. J Biol Chem 2010; 285: 13990–14004.
Gschweitl M, Ulbricht A, Barnes CA, Enchev RI, Stoffel-Studer I, Meyer-Schaller N et al. A SPOPL/Cullin-3 ubiquitin ligase complex regulates endocytic trafficking by targeting EPS15 at endosomes. Elife 2016, 5.
Mizushima N, Yoshimori T, Ohsumi Y . The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27: 107–132.
Laplante M, Sabatini DM . mTOR signaling in growth control and disease. Cell 2012; 149: 274–293.
Criollo A, Niso-Santano M, Malik SA, Michaud M, Morselli E, Marino G et al. Inhibition of autophagy by TAB2 and TAB3. EMBO J 2011; 30: 4908–4920.
Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 2009; 11: 385–396.
Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 2013; 15: 741–750.
Deretic V, Saitoh T, Akira S . Autophagy in infection, inflammation and immunity. Nat Rev Immunol 2013; 13: 722–737.
Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT . K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell 2013; 51: 283–296.
Gao D, Inuzuka H, Tan M-Kwang M, Fukushima H, Locasale Jason W, Liu P et al. mTOR drives its own activation via SCFβTrCP-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell 2011; 44: 290–303.
Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009; 325: 1134–1138.
Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013; 15: 406–416.
Shi CS, Kehrl JH . Traf6 and A20 differentially regulate TLR4-induced autophagy by affecting the ubiquitination of Beclin 1. Autophagy 2010; 6: 986–987.
Jin S, Tian S, Chen Y, Zhang C, Xie W, Xia X et al. USP19 modulates autophagy and antiviral immune responses by deubiquitinating Beclin-1. EMBO J 2016; 35: 866–880.
Xu C, Feng K, Zhao X, Huang S, Cheng Y, Qian L et al. Regulation of autophagy by E3 ubiquitin ligase RNF216 through BECN1 ubiquitination. Autophagy 2014; 10: 2239–2250.
Sorbara MT, Girardin SE . Emerging themes in bacterial autophagy. Curr Opin Microbiol 2015; 23: 163–170.
Shaid S, Brandts CH, Serve H, Dikic I . Ubiquitination and selective autophagy. Cell Death Differ 2013; 20: 21–30.
Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 2013; 501: 512–516.
Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 2015; 17: 811–819.
Watson RO, Manzanillo PS, Cox JS . Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 2012; 150: 803–815.
Huett A, Heath RJ, Begun J, Sassi SO, Baxt LA, Vyas JM et al. The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella typhimurium. Cell Host Microbe 2012; 12: 778–790.
Johansen T, Lamark T . Selective autophagy mediated by autophagic adapter proteins. Autophagy 2014; 7: 279–296.
Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F . Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012; 482: 414–418.
Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011; 333: 228–233.
Green DR, Llambi F . Cell death signaling. Cold Spring Harb Perspect Biol 2015, 7.
Wertz IE, Dixit VM . Regulation of death receptor signaling by the ubiquitin system. Cell Death Differ 2010; 17: 14–24.
Wang L, Du F, Wang X . TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008; 133: 693–703.
Mahul-Mellier AL, Pazarentzos E, Datler C, Iwasawa R, AbuAli G, Lin B et al. De-ubiquitinating protease USP2a targets RIP1 and TRAF2 to mediate cell death by TNF. Cell Death Differ 2012; 19: 891–899.
Zaman MM, Nomura T, Takagi T, Okamura T, Jin W, Shinagawa T et al. Ubiquitination-deubiquitination by the TRIM27-USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 2013; 33: 4971–4984.
Peltzer N, Rieser E, Taraborrelli L, Draber P, Darding M, Pernaute B et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep 2014; 9: 153–165.
Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep 2015; 13: 2258–2272.
Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 2006; 124: 601–613.
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009; 137: 721–735.
Gonzalvez F, Lawrence D, Yang B, Yee S, Pitti R, Marsters S et al. TRAF2 sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol Cell 2012; 48: 888–899.
Martinou J-C, Youle Richard J . Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011; 21: 92–101.
Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN et al. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol Cell 2009; 33: 109–116.
Weber A, Heinlein M, Dengjel J, Alber C, Singh PK, Hacker G . The deubiquitinase Usp27x stabilizes the BH3-only protein Bim and enhances apoptosis. EMBO Rep 2016; 17: 724–738.
Llambi F, Wang YM, Victor B, Yang M, Schneider DM, Gingras S et al. BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation. Cell 2016; 165: 421–433.
Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol 2007; 27: 4006–4017.
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011; 471: 104–109.
Zhong Q, Gao W, Du F, Wang X . Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 2005; 121: 1085–1095.
Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010; 463: 103–107.
Speidel D . Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol 2010; 20: 14–24.
Wade M, Wang YV, Wahl GM . The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol 2010; 20: 299–309.
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z . USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 2010; 140: 384–396.
Marchenko ND, Wolff S, Erster S, Becker K, Moll UM . Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J 2007; 26: 923–934.
Eckelman BP, Salvesen GS, Scott FL . Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006; 7: 988–994.
Suzuki Y, Nakabayashi Y, Takahashi R . Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA 2001; 98: 8662–8667.
Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM, Bratton SB . The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem 2009; 284: 12772–12782.
Silke J, Kratina T, Chu D, Ekert PG, Day CL, Pakusch M et al. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc Natl Acad Sci USA 2005; 102: 16182–16187.
Cambronne ED, Roy CR . Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems. Traffic 2006; 7: 929–939.
Costa TR, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 2015; 13: 343–359.
Hicks SW, Galan JE . Exploitation of eukaryotic subcellular targeting mechanisms by bacterial effectors. Nat Rev Microbiol 2013; 11: 316–326.
Shames SR, Finlay BB . Bacterial effector interplay: a new way to view effector function. Trends Microbiol 2012; 20: 214–219.
Rohde JR, Breitkreutz A, Chenal A, Sansonetti PJ, Parsot C . Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 2007; 1: 77–83.
Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C . A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat Cell Biol 2010; 12: 66–73, sup pp 61-69.
Ashida H, Nakano H, Sasakawa C . Shigella IpaH0722 E3 ubiquitin ligase effector targets TRAF2 to inhibit PKC-NF-kappaB activity in invaded epithelial cells. PLoS Pathog 2013; 9: e1003409.
Wang F, Jiang Z, Li Y, He X, Zhao J, Yang X et al. Shigella flexneri T3SS effector IpaH4.5 modulates the host inflammatory response via interaction with NF-kappaB p65 protein. Cell Microbiol 2013; 15: 474–485.
Suzuki S, Mimuro H, Kim M, Ogawa M, Ashida H, Toyotome T et al. Shigella IpaH7.8 E3 ubiquitin ligase targets glomulin and activates inflammasomes to demolish macrophages. Proc Natl Acad Sci USA 2014; 111: E4254–E4263.
Pruneda JN, Smith FD, Daurie A, Swaney DL, Villen J, Scott JD et al. E2~Ub conjugates regulate the kinase activity of Shigella effector OspG during pathogenesis. EMBO J 2014; 33: 437–449.
Sanada T, Kim M, Mimuro H, Suzuki M, Ogawa M, Oyama A et al. The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response. Nature 2012; 483: 623–626.
Steele-Mortimer O . The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol 2008; 11: 38–45.
McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V . Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol 2009; 12: 117–124.
Mesquita FS, Thomas M, Sachse M, Santos AJ, Figueira R, Holden DW . The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog 2012; 8: e1002743.
Ye Z, Petrof EO, Boone D, Claud EC, Sun J . Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol 2007; 171: 882–892.
Bernal-Bayard J, Ramos-Morales F . Salmonella type III secretion effector SlrP is an E3 ubiquitin ligase for mammalian thioredoxin. J Biol Chem 2009; 284: 27587–27595.
Haraga A, Miller SI . A Salmonella type III secretion effector interacts with the mammalian serine/threonine protein kinase PKN1. Cell Microbiol 2006; 8: 837–846.
Bhavsar AP, Brown NF, Stoepel J, Wiermer M, Martin DD, Hsu KJ et al. The Salmonella type III effector SspH2 specifically exploits the NLR co-chaperone activity of SGT1 to subvert immunity. PLoS Pathog 2013; 9: e1003518.
Lin DY, Diao J, Chen J . Crystal structures of two bacterial HECT-like E3 ligases in complex with a human E2 reveal atomic details of pathogen-host interactions. Proc Natl Acad Sci USA 2012; 109: 1925–1930.
Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D . The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol 2006; 62: 786–793.
Kubori T, Galan JE . Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell 2003; 115: 333–342.
Vonaesch P, Sellin ME, Cardini S, Singh V, Barthel M, Hardt WD . The Salmonella typhimurium effector protein SopE transiently localizes to the early SCV and contributes to intracellular replication. Cell Microbiol 2014; 16: 1723–1735.
Choi HW, Brooking-Dixon R, Neupane S, Lee CJ, Miao EA, Staats HF et al. Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity 2013; 39: 1108–1120.
Knodler LA, Winfree S, Drecktrah D, Ireland R, Steele-Mortimer O . Ubiquitination of the bacterial inositol phosphatase, SopB, regulates its biological activity at the plasma membrane. Cell Microbiol 2009; 11: 1652–1670.
Isberg RR, O'Connor TJ, Heidtman M . The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 2009; 7: 13–24.
Hubber A, Roy CR . Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 2010; 26: 261–283.
Kubori T, Hyakutake A, Nagai H . Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol 2008; 67: 1307–1319.
Price CT, Al-Quadan T, Santic M, Rosenshine I, Abu Kwaik Y . Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 2011; 334: 1553–1557.
Bruckert WM, Abu Kwaik Y . Lysine11-linked polyubiquitination of the AnkB F-Box effector of Legionella pneumophila. Infect Immun 2016; 84: 99–107.
Ensminger AW, Isberg RR . E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect Immun 2010; 78: 3905–3919.
Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H . The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol 2008; 10: 2416–2433.
Hsu F, Luo X, Qiu J, Teng YB, Jin J, Smolka MB et al. The Legionella effector SidC defines a unique family of ubiquitin ligases important for bacterial phagosomal remodeling. Proc Natl Acad Sci USA 2014; 111: 10538–10543.
Choy A, Dancourt J, Mugo B, O'Connor TJ, Isberg RR, Melia TJ et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 2012; 338: 1072–1076.
Qiu J, Sheedlo MJ, Yu K, Tan Y, Nakayasu ES, Das C et al. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 2016; 533: 120–124.
Kalita A, Hu J, Torres AG . Recent advances in adherence and invasion of pathogenic Escherichia coli. Curr Opin Infect Dis 2014; 27: 459–464.
Lin DY, Diao J, Zhou D, Chen J . Biochemical and structural studies of a HECT-like ubiquitin ligase from Escherichia coli O157:H7. J Biol Chem 2011; 286: 441–449.
Piscatelli H, Kotkar SA, McBee ME, Muthupalani S, Schauer DB, Mandrell RE et al. The EHEC type III effector NleL is an E3 ubiquitin ligase that modulates pedestal formation. PLoS One 2011; 6: e19331.
Wu B, Skarina T, Yee A, Jobin MC, Dileo R, Semesi A et al. NleG Type 3 effectors from enterohaemorrhagic Escherichia coli are U-Box E3 ubiquitin ligases. PLoS Pathog 2010; 6: e1000960.
Yan D, Quan H, Wang L, Liu F, Liu H, Chen J et al. Enteropathogenic Escherichia coli Tir recruits cellular SHP-2 through ITIM motifs to suppress host immune response. Cell Signal 2013; 25: 1887–1894.
Zhang L, Ding X, Cui J, Xu H, Chen J, Gong YN et al. Cysteine methylation disrupts ubiquitin-chain sensing in NF-kappaB activation. Nature 2012; 481: 204–208.
Cui J, Yao Q, Li S, Ding X, Lu Q, Mao H et al. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 2010; 329: 1215–1218.
Marchès O, Ledger TN, Boury M, Ohara M, Tu X, Goffaux F et al. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol 2003; 50: 1553–1567.
Tan KS, Chen Y, Lim YC, Tan GY, Liu Y, Lim YT et al. Suppression of host innate immune response by Burkholderia pseudomallei through the virulence factor TssM. J Immunol 2010; 184: 5160–5171.
Haase R, Richter K, Pfaffinger G, Courtois G, Ruckdeschel K . Yersinia outer protein P suppresses TGF- -activated kinase-1 activity to impair innate immune signaling in Yersinia enterocolitica-infected cells. J Immunol 2005; 175: 8209–8217.
Paquette N, Conlon J, Sweet C, Rus F, Wilson L, Pereira A et al. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci USA 2012; 109: 12710–12715.
Sweet CR, Conlon J, Golenbock DT, Goguen J, Silverman N . YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell Microbiol 2007; 9: 2700–2715.
Thiefes A, Wolf A, Doerrie A, Grassl GA, Matsumoto K, Autenrieth I et al. The Yersinia enterocolitica effector YopP inhibits host cell signalling by inactivating the protein kinase TAK1 in the IL-1 signalling pathway. EMBO Rep 2006; 7: 838–844.
Zhou H, Monack DM, Kayagaki N, Wertz I, Yin J, Wolf B et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J Exp Med 2005; 202: 1327–1332.
Gao X, Wang X, Pham TH, Feuerbacher LA, Lubos ML, Huang M et al. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-kappaB activation. Cell Host Microbe 2013; 13: 87–99.
Pizarro-Cerda J, Kuhbacher A, Cossart P . Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb Perspect Med 2012; 2: a010009.
Bonazzi M, Veiga E, Pizarro-Cerda J, Cossart P . Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol 2008; 10: 2208–2222.
Veiga E, Cossart P . Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 2005; 7: 894–900.
Gouin E, Adib-Conquy M, Balestrino D, Nahori MA, Villiers V, Colland F et al. The Listeria monocytogenes InlC protein interferes with innate immune responses by targeting the I{kappa}B kinase subunit IKK{alpha}. Proc Natl Acad Sci USA 2010; 107: 17333–17338.
Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol 2009; 11: 1233–1240.
Ribet D, Hamon M, Gouin E, Nahori MA, Impens F, Neyret-Kahn H et al Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 2010; 464: 1192–1195.
O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP . The immune response in tuberculosis. Annu Rev Immunol 2013; 31: 475–527.
Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH . Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 2008; 322: 1104–1107.
Ruggiero A, Squeglia F, Romano M, Vitagliano L, De Simone A, Berisio R . The structure of resuscitation promoting factor B from M. tuberculosis reveals unexpected ubiquitin-like domains. Biochim Biophys Acta 2016; 1860: 445–451.
Chen Z . Mycobacterium tuberculosis favors its survival by utilizing host ubiquitin to impair innate immunity. Natl Sci Rev 2015; 2: 260–261.
Wang J, Li BX, Ge PP, Li J, Wang Q, Gao GF et al. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat Immunol 2015; 16: 237–245.
Guler R, Brombacher F . Host-directed drug therapy for tuberculosis. Nat Chem Biol 2015; 11: 748–751.
Acknowledgements
We acknowledge research funding from the National Basic Research Programs of China (Grant Nos 2012CB518700 and 2014CB744400), the National Natural Science Foundation of China (Grant Nos 81371769 and 81571954) and the Youth Innovation Promotion Association CAS (Grant No. Y12A027BB2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Chai, QY. & Liu, C. The ubiquitin system: a critical regulator of innate immunity and pathogen–host interactions. Cell Mol Immunol 13, 560–576 (2016). https://doi.org/10.1038/cmi.2016.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.40
Keywords
This article is cited by
-
In search of the Aplysia immunome: an in silico study
BMC Genomics (2022)
-
Ubiquitin-related processes and innate immunity in C. elegans
Cellular and Molecular Life Sciences (2021)
-
New insights into the evasion of host innate immunity by Mycobacterium tuberculosis
Cellular & Molecular Immunology (2020)
-
Host defense mechanisms against Mycobacterium tuberculosis
Cellular and Molecular Life Sciences (2020)
-
Mycobacterium tuberculosis Mce2E suppresses the macrophage innate immune response and promotes epithelial cell proliferation
Cellular & Molecular Immunology (2019)