Abstract
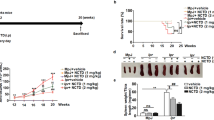
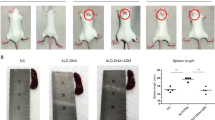
We previously reported that SM934, a water-soluble artemisinin derivative, was a viable treatment in murine lupus models. In the current study, we further investigated the therapeutic effects of a modified dosage regimen of SM934 on lupus-prone MRL/lpr mice and explored its effects on B cell responses, a central pathogenic event in systemic lupus erythematosus (SLE). When orally administered twice-daily, SM934 significantly prolonged the life-span of MRL/lpr mice, ameliorated the lymphadenopathy symptoms and decreased the levels of serum anti-nuclear antibodies (ANAs) and of the pathogenic cytokines IL-6, IL-10 and IL-21. Furthermore, SM934 treatment restored the B-cell compartment in the spleen of MRL/lpr mice by increasing quiescent B cell numbers, maintaining germinal center B-cell numbers, decreasing activated B cell numbers and reducing plasma cell (PC) numbers. Ex vivo, SM934 suppressed the Toll-like receptor (TLR)-triggered activation and proliferation of B cells, as well as antibody secretion. Moreover, the present study demonstrated that SM934 interfered with the B-cell intrinsic pathway by downregulating TLR7/9 mRNA expression, MyD88 protein expression and NF-κB phosphorylation. In human peripheral blood mononuclear cells (PBMCs), consistent with the results in MRL/lpr mice, SM934 inhibited TLR-associated B-cell activation and PC differentiation. In conclusion, a twice daily dosing regimen of SM934 had therapeutic effects on lupus-prone MRL/lpr mice by suppressing B cell activation and plasma cell formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Theofilopoulos AN, Dixon FJ . Murine models of systemic lupus erythematosus. Adv Immunol 1985; 37: 269–390.
Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62.
Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM . BCL-6 Represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000; 13: 199–212.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277.
Egner W . The use of laboratory tests in the diagnosis of SLE. J Clin Pathol 2000; 53: 424–432.
Muro Y . Antinuclear antibodies. Autoimmunity 2005; 38: 3–9.
Hua Z, Hou B . TLR signaling in B-cell development and activation. Cell Mol Immunol 2012; 10: 103–106.
Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A . Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002; 416: 603–607.
Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 2005; 202: 1171–1177.
Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ . Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity 2013; 38: 528–540.
Robak E, Sysa-Jedrzejowska A, Stepien H, Robak T . Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Network 1997; 8: 281–286.
Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 1995; 181: 839–844.
Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K . IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R. Fc reduces disease progression. J Immunol 2007; 178: 3822–3830.
Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–5371.
Deng XM, Yan SX, Wei W . IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation. Cell Mol Immunol 2015; 21: 31–39.
Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Faslpr/lpr/J mice. J Immunol 2012; 188: 1656–1667.
Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007; 27: 461–466.
Lu L . [Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2002; 22: 169–171. Chinese.
Jin O, Zhang H, Gu Z, Zhao S, Xu T, Zhou K et al. A pilot study of the therapeutic efficacy and mechanism of artesunate in the MRL/lpr murine model of systemic lupus erythematosus. Cell Mol Immunol 2009; 6: 461–467.
Wu X, Zhang W, Shi X, An P, Sun W, Wang Z . Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms. Acta Biochim Biophys Sin 2010; 42: 916–923.
Hou LF, He SJ, Wang JX, Yang Y, Zhu FH, Zhou Y et al. SM934, a water-soluble derivative of arteminisin, exerts immunosuppressive functions in vitro and in vivo. Int Immunopharmacol 2009; 9: 1509–1517.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–2455.
Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH et al. SM934 treated lupus-prone NZB×NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One 2012; 7: e32424.
Monahan E, Yamazaki K . An improved urine collection technique for laboratory mice: the bladder massage method. Lab Animal (USA) 1993; 38–39.
Yang LY, Chen A, Kuo YC, Lin CY . Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med 1999; 134: 492–500.
Tao X, Fan F, Hoffmann V, Longo NS, Lipsky PE . Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res Ther 2006; 8: R24.
Tao X, Fan F, Hoffmann V, Gao CY, Longo NS, Zerfas P et al. Effective therapy for nephritis in (NZB×NZW) F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum 2008; 58: 1774–1783.
Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 2008; 58: 1433–1444.
Tan X, Fan F, Hoffman V, Longo NS, Lipsky PE . Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. FASEB J 2006; 20: A1147.
He SJ, Lin ZM, Wu YW, Bai BX, Yang XQ, He PL et al. Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZB×NZW F1 mice via interference with TLR-mediated APC response. Acta Pharmacol Sin 2014; 35: 219–229.
Avalos AM, Busconi L, Marshak-Rothstein A . Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity 2010; 43: 76–83.
Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell Richard A, Shlomchik MJ . Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006; 25: 417–428.
Marshak-Rothstein A . Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6: 823–835.
Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 2000; 165: 5970–5979.
Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum 2007; 56: 1618–1628.
Xu L, Chen X, Tu Y . Efect of hydroartemisinin on lupus BXSB mice. Chin J Dermatovenerol Integr Tradit West Med 2002; 1: 19–20.
Hou L, Block KE, Huang H . Artesunate abolishes germinal center B cells and inhibits autoimmune arthritis. PLoS One 2014; 9: e104762.
Ditzel HJ . The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med 2004; 10: 40–45.
William J, Euler C, Christensen S, Shlomchik MJ . Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002; 297: 2066–2070.
Aldieri E, Atragene D, Bergandi L, Riganti C, Costamagna C, Bosia A et al. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-κB activation. FEBS Lett 2003; 552: 141–144.
Li WD, Dong YJ, Tu YY, Lin ZB . Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharmacol 2006; 6: 1243–1250.
Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V . Increased frequency of pre-germinal center b cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol 2001; 167: 2361–2369.
Shlomchik MJ, Euler CW, Christensen SC, William J . Activation of rheumatoid factor (RF) B cells and somatic hypermutation outside of germinal centers in autoimmune-prone MRL/lpr mice. Ann NY Acad Sci 2003; 987: 38–50.
William J, Euler C, Shlomchik MJ . Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol 2005; 174: 6879–6887.
Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A . Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765.
Gröndal G, Gunnarsson I, Rönnelid J, Rogberg S, Klareskog L, Lundberg I . Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 1999; 18: 565–570.
Park Y, Lee S, Kim D, Lee J, Lee C, Song C . Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 1997; 16: 283–288.
Acknowledgements
This work was supported by grants from the National Science Fair Committee (NSFC), China (No. 81273524, 81322049), National Science & Technology Major Project ‘New Drug Creation and Manufacturing Program’, China (2014ZX09101002) and National Key Basic Research Programme (973 Programme, 2014CB541906). We are also grateful to Dr Hou Lifei who kindly contributed to the manuscript revision.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wu, Y., He, S., Bai, B. et al. Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation. Cell Mol Immunol 13, 379–390 (2016). https://doi.org/10.1038/cmi.2015.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2015.13
Keywords
This article is cited by
-
Role of sex in immune response and epigenetic mechanisms
Epigenetics & Chromatin (2024)
-
Triptolide regulates the balance of Tfr/Tfh in lupus mice
Advances in Rheumatology (2023)
-
Mitochondrial DNA-Sensing Pathogen Recognition Receptors in Systemic Sclerosis-Associated Interstitial Lung Disease: a Review
Current Treatment Options in Rheumatology (2023)
-
Artemisinin derivative SM934 in the treatment of autoimmune and inflammatory diseases: therapeutic effects and molecular mechanisms
Acta Pharmacologica Sinica (2022)
-
Neuronal NR4A1 deficiency drives complement-coordinated synaptic stripping by microglia in a mouse model of lupus
Signal Transduction and Targeted Therapy (2022)