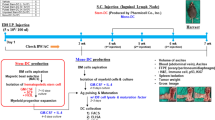
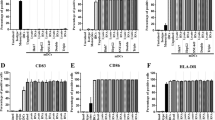
Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting cellular tumor vaccines contribute to the induction of potent antitumor immune responses in murine models and patients suffering from cancers. Hepatocellular carcinoma (HCC) is one of the most frequent and malignant cancers in China. We describe, for the first time, a GM-CSF releasing vaccine strategy that represents a step toward combating this type of cancer. In this study, a bystander cell-based GM-CSF secreting vaccine against murine HCC, Hepa1–6/B78H1-GM-CSF, was co-administered with a low dose of cyclophosphamide (CY). After challenging with tumor and vaccination, immunological assays demonstrated that the cellular antitumor immune responses were efficiently activated and that tumor development was significantly retarded, which was dependent on synergy with CY. The promising outcome of the anti-HCC vaccine in the murine model demonstrates the feasibility of a future clinical application for this treatment in HCC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kishi Y, Hasegawa K, Sugawara Y, Kokudo N . Hepatocellular carcinoma: current management and future development-improved outcomes with surgical resection. Int J Hepatol 2011; 2011: 728103.
Hanish SI, Knechtle SJ . Liver transplantation for the treatment of hepatocellular carcinoma. Oncology 2011; 25: 752–757.
Hu Z, Zhao W . Novel insights into the molecular mechanisms of alpha-fetoprotein expression and malignant phenotypes of hepatocellular carcinoma. Cell Mol Immunol 2012; 9: 7–8.
Wang L, Zhang Z, Wang FS . The efficacy of miRNA122, a novel therapeutic target, for predicting the progression of hepatocellular carcinoma (HCC). Cell Mol Immunol 2012; 9: 103–104.
Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19: 145–156.
Barrio MM, de Motta PT, Kaplan J, von Euw EM, Bravo AI, Chacon RD et al. A phase I study of an allogeneic cell vaccine (VACCIMEL) with GM-CSF in melanoma patients. J Immunother 2006; 29: 444–454.
Emens LA, Armstrong D, Biedrzycki B, Davidson N, Davis-Sproul J, Fetting J et al. A phase I vaccine safety and chemotherapy dose-finding trial of an allogeneic GM-CSF-secreting breast cancer vaccine given in a specifically timed sequence with immunomodulatory doses of cyclophosphamide and doxorubicin. Hum Gene Ther 2004; 15: 313–337.
Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol 2009; 27: 5911–5918.
Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008; 14: 1455–1463.
Abe Y, Ito T, Baba E, Nagafuji K, Kawabe K, Choi I et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation as immunotherapy for pancreatic cancer. Pancreas 2009; 38: 815–819.
Emens LA, Jaffee EM . Cancer vaccines: an old idea comes of age. Cancer Biol Ther 2003; 2 (4 Suppl 1): S161–S168.
Nemunaitis J, Sterman D, Jablons D, Smith JW 2nd, Fox B, Maples P et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004; 96: 326–331.
Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006; 13: 555–562.
Smith BD, Kasamon YL, Kowalski J, Gocke C, Murphy K, Miller CB et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res 2010; 16: 338–347.
Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S . CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 2005; 106: 1008–1011.
Fehervari Z, Sakaguchi S . CD4+ Tregs and immune control. J Clin Invest 2004; 114: 1209–1217.
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B . Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003; 9: 606–612.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949.
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169: 2756–2761.
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E . Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59: 3128–3133.
Berd D, Maguire HC Jr, Mastrangelo MJ . Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res 1986; 46: 2572–2527.
Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res 2001; 61: 3689–3697.
Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP et al. Recruitment of latent pools of high-avidity CD8+ T cells to the antitumor immune response. J Exp Med 2005; 201: 1591–1602.
Borrello I, Sotomayor EM, Cooke S, Levitsky HI . A universal granulocyte-macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum Gene Ther 1999; 10: 1983–1991.
Jaffee EM, Thomas MC, Huang AY, Hauda KM, Levitsky HI, Pardoll DM . Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J Immunother Emphasis Tumor Immunol 1996; 19: 176–183.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I . High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 2004; 64: 6337–6343.
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H . The central role of CD4+ T cells in the antitumor immune response. J Exp Med 1998; 188: 2357–2368.
Serre K, Mohr E, Gaspal F, Lane PJ, Bird R, Cunningham AF et al. IL-4 directs both CD4 and CD8 T cells to produce Th2 cytokines in vitro, but only CD4 T cells produce these cytokines in response to alum-precipitated protein in vivo. Mol Immunol 2010; 47: 1914–1922.
Hogan SP, Koskinen A, Matthaei KI, Young IG, Foster PS . Interleukin-5-producing CD4+ T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am J Respir Crit Care Med 1998; 157: 210–218.
Qin Z, Blankenstein T . CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity 2000; 12: 677–686.
Kabashima H, Nagata K, Maeda K, Iijima T . Interferon-gamma-producing cells and inducible nitric oxide synthase-producing cells in periapical granulomas. J Oral Pathology Med 1998; 27: 95–100.
Hahn S, Gehri R, Erb P . Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev 1995; 146: 57–79.
Tsukui T, Hildesheim A, Schiffman MH, Lucci J 3 rd, Contois D, Lawler P et al. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res 1996; 56: 3967–3974.
Dou AX, Feng LL, Liu XQ, Wang X . Cyclic adenosine monophosphate involvement in low-dose cyclophosphamide-reversed immune evasion in a mouse lymphoma model. Cell Mol Immunol 2012; 9: 482–488.
Emens LA . Chemoimmunotherapy. Cancer J 2010; 16: 295–303.
Acknowledgements
This work was supported by grants from National Basic Research Program of China (2012CB910800 to BS), the National Natural Science Foundation (81072029 to BS and 30901750, 81272322 to YC, 81201528 to RJ), Major Research Plan of the National Natural Science Foundation (91029721 to BS), Natural Science Foundation of Jiangsu Province (BK2010532 to YC), China Postdoctoral Science Foundation-funded project (20090461133 to YC), Jiangsu Planned Projects for Postdoctoral Research Funds (1001028B to YC), Jiangsu Province Laboratory of Pathogen Biology (11BYKF02 to QS), Ministry of Health Research Foundation, China (LW201001 to LD) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The work was also supported in part by the Program for Development of Innovative Research Teams in the First Affiliated Hospital of NJMU.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
All authors have contributed significantly and declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, C., Hou, J., Lin, Z. et al. A bystander cell-based GM-CSF secreting vaccine synergized with a low dose of cyclophosphamide presents therapeutic immune responses against murine hepatocellular carcinoma. Cell Mol Immunol 10, 349–359 (2013). https://doi.org/10.1038/cmi.2013.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2013.20