Abstract
γδ-T cells play an indispensable role in host defense against different viruses, including influenza A virus. However, whether these cells have cytotoxic activity against influenza virus-infected lung alveolar epithelial cells and subsequently contribute to virus clearance remains unknown. Using influenza virus-infected A549 cells, human lung alveolar epithelial cells, we investigated the cytotoxic activity of aminobisphosphonate pamidronate (PAM)-expanded human Vγ9Vδ2-T cells and their underlying mechanisms. We found that PAM could selectively activate and expand human Vγ9Vδ2-T cells. PAM-expanded human Vγ9Vδ2-T cells efficiently killed influenza virus-infected lung alveolar epithelial cells and inhibited virus replication. The cytotoxic activity of PAM-expanded Vγ9Vδ2-T cells was dependent on cell-to-cell contact and required NKG2D activation. Perforin–granzyme B, tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas–Fas ligand (FasL) pathways were involved in their cytotoxicity. Our study suggests that targeting γδ-T cells by PAM can potentially offer an alternative option for the treatment of influenza virus.
Similar content being viewed by others
Introduction
Although they represent only a small population of immune cells, γδ-T cells exhibit features characteristic of both innate and adaptive immunity and play an indispensable role in host defense, immune surveillance and homeostasis.1,2,3,4 In humans, γδ-T cells constitute approximately 1%–5% of circulating T cells, and most of them bear the Vγ9Vδ2 T-cell receptor (TCR).5,6 Human Vγ9Vδ2-T cells can be specifically activated in an HLA-unrestricted manner by small non-peptidic phosphoantigens, which are metabolites of the isoprenoid biosynthesis pathways.7 It is known that isopentenyl pyrophosphate (IPP), an intermediate produced through the mevalonate pathway, can selectively activate and expand human Vγ9Vδ2-T cells.8,9,10 Pharmacological compounds, such as the aminobisphosphonate pamidronate (PAM), which is commonly used for the treatment of osteoporosis, can activate and expand human Vγ9Vδ2-T cells by inducing the intracellular accumulation of IPP.8,11
γδ-T cells are broadly reactive against different viruses,12,13,14,15,16,17,18 such as herpes viruses (herpes simplex virus, cytomegalovirus, human herpes virus-6), vaccinia virus, influenza virus, coxsackie B virus and human or simian immunodeficiency virus (HIV/SIV), indicating a role for these lymphocytes in antiviral immune responses. Cell-mediated cytotoxicity is the major mechanism to eliminate virus-infected cells and thus to eliminate potential sources of new virus. Previously, using influenza virus-infected human monocyte-derived macrophages, we showed that IPP-expanded human Vγ9Vδ2-T cells had cytotoxic activity against virus-infected monocyte-derived macrophages.10 However, macrophages are not the major target cells for influenza virus infection, despite the influenza virus' ability to replicate in human macrophages.19 Human lung alveolar epithelial cells, as the major target cells for influenza virus infection, are a more reliable target to investigate the antiviral role of γδ-T cells. Recently, we demonstrated that IPP-expanded human Vγ9Vδ2-T cells had a non-cytolytic effect on human influenza virus by releasing IFN-γ in virus-infected lung alveolar epithelial cells.20 However, whether these cells have cytolytic effects in influenza virus-infected lung alveolar epithelial cells, and subsequently contribute to virus clearance, is still unknown.
In this study, using influenza virus-infected human lung alveolar epithelial cells, we investigated the cytotoxic activity of PAM-expanded human Vγ9Vδ2-T cells and their underlying mechanisms. We found that PAM-expanded human Vγ9Vδ2-T cells efficiently killed influenza virus-infected lung alveolar epithelial cells and inhibited virus replication. The cytotoxic activity of PAM-expanded Vγ9Vδ2-T cells was dependent on cell-to-cell contact and required NKG2D activation. The pathways of perforin–granzyme B, tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas–Fas ligand (FasL) mediated their cytotoxicity.
Materials and methods
Cells
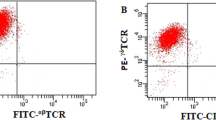
Human peripheral blood was obtained from healthy donors in accordance with the Hong Kong University Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ, USA) gradient centrifugation, as previously described.21 PBMCs were cultured in 10% fetal bovine serum RPMI-1640 medium with 9 µg/ml of PAM. Recombinant human IL-2 (Invitrogen, Carlsbad, CA, USA) was added to reach a final concentration of 500 IU/ml every 3 days from day 3. After being cultured for 20 days, Vγ9Vδ2-T cells were purified by negative selection with a TCR γδ-T-cell isolation kit, according to the manufacturer's instruction (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of γδ-T cells, as determined by flow cytometry, was consistently >97%. Immortalized human alveolar type II epithelial cells (A549) were cultured in RPMI 1640 medium supplemented with 10% bovine calf serum.
Virus preparation, titration and infection
Influenza H1N1 virus (A/PR/8/34) was used. The virus was cultured in Madin–Darby canine kidney cells as previously described.22,23 The virus titer was determined by daily observation of the cytopathic effect in cells infected with serial dilutions of virus stock, and the median tissue culture infective dose (TCID50) was calculated according to the Reed–Muench formula. A549 cells were infected by influenza virus at a multiplicity of infection (MOI) of 2. After 1 h of viral adsorption, the cells were washed by phosphate-buffered saline to remove unadsorbed virus.
Flow cytometry
Cells were stained for surface markers with the following antibodies: anti-CD3, anti-TCRγ9, anti-TCRδ2, anti-NKG2D, anti-CD69, anti-TRAIL, anti-TCR-γδ, anti-MICA/B, anti-Fas, anti-FasL, anti-TRAIL and their ligands DR4 and DR5. For intracellular staining, cells were fixed, permeabilized and then stained with anti-perforin and anti-granzyme B (GrB, GB11) antibodies or their relevant isotype controls. All samples were acquired by flow cytometry and analyzed by FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Cytotoxicity assay
Purified Vγ9Vδ2-T cells (effector) were cocultured with H1N1 virus-infected or mock-infected A549 cells (target) at different effector-to-target (E/T) ratios for 5–6 h. Afterward, non-adherent cells were harvested directly. Adherent cells were detached with 0.25% trypsin-ethylenediaminetetraacetic acid. All of the adherent and non-adherent cells were then stained with anti-human CD3 to identify Vγ9Vδ2-T cells and ethidium homodimer-2 (EthD-2) to identify dead cells. The cytotoxicity of Vγ9Vδ2-T cells against A549 cells was assessed by flow cytometry as the percentage of EthD-2+ cells in the CD3− population, as previously described.10
Transwell coculture
To evaluate the cell–cell contact requirement for Vγ9Vδ2-T-cell cytotoxicity, a transwell culture system (24-well, pore size 0.4 µm; Millipore, Bedford, MA, USA) was used. A549 cells (Target, T) in the bottom well were infected with influenza virus at an MOI of 2, and Vγ9Vδ2-T cells (Effector, E) were added directly into the bottom wells or into transwell inserts at an E/T ratio of 10∶1. After culturing for 5 h, the A549 cells in the bottom wells were harvested and analyzed for cell death, as described above. The supernatants in the transwell coculture of Vγ9Vδ2-T cells and A549s were collected at the indicated time for determining the virus titer, as described above.
Blocking assay
Purified Vγ9Vδ2-T cells (E) were cocultured with influenza virus-infected A549 cells (T) at an E/T ratio of 10∶1 for 5 h. The neutralization antibodies anti-NKG2D (10 µg/ml; BD Biosciences, San Jose, CA, USA), anti-FasL (10 µg/ml; R&D, Minneapolis, MN, USA), anti-TRAIL (10 µg/ml; R&D) and their relevant isotype control mouse IgG1 (mIgG1) were added to the coculture for blocking NKG2D, FasL and TRAIL mediated pathways, respectively. For blocking perforin and granzyme B, the perforin inhibitor concanamycin A (CMA) (1 µg/ml; Sigma, St Louis, MO, USA) and granzyme B inactivator Bcl-2 (1 µg/ml; R&D) were used, as in previous reports.24 The cytotoxicities were analyzed by flow cytometry as described above and calculated as % inhibition relative to those of the control.
Statistical analysis
Data are expressed as the mean±standard errors of the mean. Statistical significance was determined by the Student's t-test or nonparametric tests using Graphpad Prism software (version 5). A P value of <0.05 was considered to be significant.
Results
PAM selectively activates and expands human Vγ9Vδ2-T cells
Similar to IPP,25,26 PAM and IL-2 can also selectively expand human Vγ9Vδ2-T cells. Freshly isolated PBMCs contained 1%–6% of Vγ9Vδ2-T cells from 16 randomly selected healthy adult donors; after 20 days of culture in the presence of PAM and IL-2, the percentage of Vγ9Vδ2-T cells within PBMC increased to 85%–97% and the absolute numbers of the Vγ9Vδ2-T cells were significantly increased by 93-fold (range: 54–151-fold). In contrast, IL-2 alone did not increase the absolute number of Vγ9Vδ2-T cells.
To determine whether PAM can activate human Vγ9Vδ2-T cells, we examined cell surface markers (CD69, NKG2D, Fas, FasL and TRAIL) and intracellular cytolytic granules (perforin and granzyme B) in fresh and PAM-expanded Vγ9Vδ2-T cells. As shown in Figure 1, fresh Vγ9Vδ2-T cells expressed low levels of FasL, high levels of Fas and medium levels of CD69, NKG2D, TRAIL, perforin and granzyme B. In contrast, PAM-expanded Vγ9Vδ2-T cells had much higher levels of CD69, NKG2D, FasL, TRAIL, perforin and granzyme B expression compared to fresh Vγ9Vδ2-T cells (Figure 1).
Phenotypes of fresh and PAM-expanded Vγ9Vδ2-T human cells. The white histograms represent the surface expression of CD69, NKG2D, MIC A/B, Fas, FasL, TRAIL, DR4 (TRAIL receptor 1), DR5 (TRAIL receptor 2), intracellular perforin and granzyme B, and the gray histograms represent isotype controls. Data shown here are representative of four separate experiments. FasL, Fas–Fas ligand; PAM, aminobisphosphonate pamidronate; TRAIL, tumor-necrosis factor-related apoptosis-inducing ligand.
PAM-expanded Vγ9Vδ2-T cells efficiently kill influenza virus-infected A549 cells
To determine the cytotoxic activity of Vγ9Vδ2-T cells against influenza virus-infected A549 cells, purified PAM-expanded Vγ9Vδ2-T cells were cocultured with mock- or influenza virus-infected A549 cells for 5 h. As shown in Figure 2, Vγ9Vδ2-T cells displayed cytotoxic activity against both mock- and virus-infected A549s in a dose-dependent manner. Importantly, the killing of Vγ9Vδ2-T cells against influenza virus-infected A549 cells significantly increased compared to that against mock-treated A549 cells at E/T ratios of 10∶1 or 20∶1. These results demonstrate that PAM-expanded Vγ9Vδ2-T cells have potent cytotoxic activity against influenza virus-infected lung alveolar epithelial cells.
PAM-expanded Vγ9Vδ2-T cells efficiently killed influenza virus-infected A549 cells. A549 cells (Target, T) were either mock infected (A549) or infected with influenza H1N1 PR/8 virus at an MOI of 2 (vA549), and then cultured with purified PAM-expanded Vγ9Vδ2 T cells (Effector, E) at various E/T ratios for 5 h. The percentages (mean±s.e.m.) of dead A549 cells among target cells (CD3− population), identified as CD3−EthD2+, for four different experiments are shown. *P<0.05, **P<0.01. MOI, multiplicity of infection; PAM, aminobisphosphonate pamidronate.
The killing of virus-infected A549 cells by Vγ9Vδ2-T cells is dependent on cell-to-cell contact
To clarify whether the killing of influenza virus-infected A549 cells by Vγ9Vδ2-T cells requires cell-to-cell contact, we applied a transwell culture system to separate virus-infected A549 cells from Vγ9Vδ2-T cells at an E/T ratio of 20∶1. As shown in Figure 3, Vγ9Vδ2-T cells lost their cytolytic activity against influenza virus-infected A549 cells when the physical contact between Vγ9Vδ2-T cells and virus-infected A549 cells was abrogated by a semipermeable membrane. These results indicate that the killing of influenza virus-infected lung alveolar epithelial cells by Vγ9Vδ2-T cells is dependent on cell-to-cell contact.
Physical contact was required for cytotoxicity of Vγ9Vδ2-T cells against influenza virus-infected A549s. A549 cells (Target, T) were infected (vA549) with influenza H1N1 PR/8 virus at an MOI of 2 and then cultured with or physically separated from the purified PAM-expanded Vγ9Vδ2 T cells (Effector, E) at E/T ratios of 20∶1 for 6 h. The percentages (mean±s.e.m.) of dead A549 cells among target cells (CD3− population), identified as CD3−EthD2+, for four different experiments are shown. *P<0.05. MOI, multiplicity of infection; PAM, aminobisphosphonate pamidronate.
Influenza virus replication in A549 cells is inhibited by Vγ9Vδ2-T cells
Influenza virus efficiently replicated within A549 cells. The virus titer in the culture supernatants gradually increased from 6 h post-infection and reached a peak level at 72 h post-infection (Figure 4). To determine whether there was a decline of the viral titer in A549 cells after coculture with Vγ9Vδ2-T cells, virus-infected A549 cells were cultured alone or with expanded Vγ9Vδ2-T cells, either in direct contact with or separate from Vγ9Vδ2-T cells. When separated from Vγ9Vδ2-T cells, culturing was performed by a transwell culture system at an E/T ratio of 20∶1. As shown in Figure 4, the virus titers in supernatant culture were significantly reduced after 24, 48 and 72 h of coculture of virus-infected A549 cells and Vγ9Vδ2-T cells, compared to virus-infected A549 cells alone. In contrast, there was no significant decrease in virus titers when Vγ9Vδ2-T cells were separated from virus-infected A549 cells (Figure 4). These data suggest that the inhibition of influenza virus replication by Vγ9Vδ2-T cells requires cell-to-cell contact.
Influenza virus replication in A549 cells was inhibited by Vγ9Vδ2-T cells. A549 cells (Target, T) were infected (vA549) with influenza H1N1 PR/8 virus at an MOI of 2 and then cultured with or physically separated from the purified PAM-expanded Vγ9Vδ2 T cells (Effector, E) at E/T ratios of 20∶1 for the indicated time. The virus titers of supernatants were determined by TCID50 on MDCK cells. Data are the mean±s.e.m. of TCID50 titers of four separate experiments. MOI, multiplicity of infection; PAM, aminobisphosphonate pamidronate.
Mechanisms underlying the cytotoxicity of Vγ9Vδ2-T cells
As PAM-expanded Vγ9Vδ2-T cells expressed high or medium levels of NKG2D, Fas, FasL and TRAIL, we further determined whether their relevant ligands were expressed in influenza virus-infected A549 cells. As shown in Figure 5a, A549 cells expressed high levels of NKG2D ligands (stress-inducible MHC class I-related proteins A and B, MAC A/B),27 Fas and TRAIL receptors (DR4 and DR5),28 but very low levels of FasL. The expressions of MIC A/B and DR5 were upregulated in A549 cells after influenza virus infection. In contrast, there were no significant changes in expressions of Fas, FasL and DR4 in A549 cells after influenza virus infection.
Mechanisms underlying the cytotoxicity of Vγ9Vδ2-T cells. (a) Phenotype of influenza virus-infected A549 cells. A549 cells were infected with influenza H1N1 PR/8 virus (MOI=2) and then cultured for 5 h. The expression level of surface MICA/B, Fas, FasL and DR4, DR5 on the virus infected-A549 cells were analyzed by flow cytometry. Data shown here are representative of four separate experiments. (b) PAM-expanded Vγ9Vδ2-T cells were cocultured with influenza PR/8 virus-infected A549 cells at a ratio of 10∶1 for 5 h. The perforin inhibitor CMA, granzyme B inactivator Bcl-2, anti-FasL, anti-TRAIL anti-NKG2D blocking antibodies, or their relevant isotype control (mouse IgG1, mIgG1), were used. The cytotoxicity was analyzed by flow cytometry as the percentage of EthD-2+ cells in the CD3− population and calculated as % inhibition relative to those without any treatment. The data shown as the mean±s.e.m. are representative of four independent experiments. **P<0.01 compared to their relevant isotype control. CMA, concanamycin A; FasL, Fas–Fas ligand; MOI, multiplicity of infection; PAM, aminobisphosphonate pamidronate; TRAIL, tumor-necrosis factor-related apoptosis-inducing ligand.
To further determine whether NKG2D, Fas–FasL and TRAIL pathways were involved in the cytotoxicity of Vγ9Vδ2-T cells, neutralizing antibodies for NKG2D, FasL and TRAIL were used. As shown in Figure 5, blockades of NKG2D, TRAIL or FasL significantly inhibited the cytolytic activities of Vγ9Vδ2-T cells against influenza virus-infected A549 cells. These results indicate that NKG2D, TRAIL and Fas–FasL pathways are involved in the killing of influenza virus-infected lung alveolar epithelial cells by Vγ9Vδ2-T cells.
To confirm the involvement of cytolytic granule release in the killing of virus-infected A549 cells by Vγ9Vδ2-T cells, the perforin specific inhibitor CMA and granzyme B inactivator Bcl-2 were used. As shown in Figure 5, the cytolytic activities of Vγ9Vδ2-T cells against influenza virus-infected A549 cells were significantly abrogated after Bcl-2 treatment or CMA treatment. These results demonstrate that the perforin–granzyme B pathway is involved in the cytotoxicity of Vγ9Vδ2-T cells against influenza virus-infected lung alveolar epithelial cells.
Discussion
In this study, using influenza virus-infected lung alveolar epithelial cells as the model, we have demonstrated for the first time that PAM-expanded Vγ9Vδ2-T cells can efficiently kill virus-infected lung alveolar epithelial cells and thus contribute to virus clearance. Our study suggests a novel therapeutic approach of using PAM to activate and expand human Vγ9Vδ2-T cells against influenza virus infection.
We showed that similar to IPP,25,26 PAM is a potent stimulator for the activation and expansion of human Vγ9Vδ2-T cells from PBMC in the presence of IL-2. Upon PAM stimulation, resting Vγ9Vδ2-T cells can be activated, as evidenced by the upregulated expressions of CD68, NKG2D, FasL and TRAIL. Importantly, during 20 days of stimulation by PAM and IL-2, Vγ9Vδ2-T cells can be largely expanded by approximately 93-fold, suggesting that PAM could be used for large-scale expansion of functional γδ-T cells in vitro for adoptive immunotherapy in influenza virus infections.
A concern for γδ-T cell-based immunotherapy is whether PAM-expanded Vγ9Vδ2-T cells can traffic to the lung, the primary infection site, during an influenza infection. Indeed, more recently, we have shown that the in vitro PAM-expanded Vγ9Vδ2-T cells can migrate to the lung and control influenza disease in immunodeficient mice.11 In addition, in a humanized mouse model, we further demonstrated that PAM can activate and expand Vγ9Vδ2-T cells in vivo, and then control human and avian influenza virus infections.11 Therefore, PAM could be an alternative option for the treatment of influenza virus infection by targeting Vγ9Vδ2-T cells.
The antiviral mechanisms of Vγ9Vδ2-T cells against different viruses are different. For examples, human Vγ9Vδ2-T cells have cytolytic activities against CMV- and herpes simplex virus-infected cells in an HLA-unrestricted manner in vitro.12,13,29 In addition to killing HIV-infected cells, Vγ9Vδ2-T cells can also block HIV entry through the coreceptor CCR5 by releasing certain CCR5-ligand chemokines.17,30 For the hepatitis C virus, Vγ9Vδ2-T cells can induce non-cytolytic inhibition of virus replication through the secretion of IFN-γ.25 Previously, we also demonstrated that IPP-expanded Vγ9Vδ2-T cells can inhibit human influenza H1N1 virus replication by releasing IFN-γ.20 Here, we further found that PAM-expanded Vγ9Vδ2-T cells can kill influenza virus-infected lung alveolar epithelial cells. Thus, our results indicate that PAM-expanded Vγ9Vδ2-T cells can control influenza virus infection through both cytolytic and non-cytolytic mechanisms.
NKG2D is a potent costimulatory receptor of the cytotoxic functions of human natural killer and Vγ9Vδ2-T cells.31 It can trigger Vγ9Vδ2-T cells to release cytolytic granules by recognition of the NKG2D ligand.32 MICA/B, as the ligand for NKG2D, was found to be upregulated in lung alveolar epithelial cells after influenza virus infection in the current study. In addition, we showed that almost all the PAM-expanded Vγ9Vδ2-T cells expressed NKG2D, and the cytotoxicity of Vγ9Vδ2-T cells against influenza virus-infected lung alveolar epithelial was significantly blocked by NKG2D neutralizing antibody, indicating the killing of influenza virus-infected cells by Vγ9Vδ2-T cells requires NKG2D activation and recognition.
Previously, we found that the TRAIL pathway was not involved in the killing of influenza virus-infected macrophages.10 However, here we found that the blockade of TRAIL by its neutralizing antibody significantly decreased the cytolytic activity of Vγ9Vδ2-T cells against influenza virus-infected lung alveolar epithelial cells. This may be due to the different expression levels of TRAIL receptors; we found that influenza virus-infected lung alveolar epithelial cells expressed high levels of DR4 and DR5, the receptors of TRAIL, whereas virus-infected macrophages only expressed very low levels of DR4 and DR5 (data not shown).
It has been shown that the Fas/FasL-mediated pathway was also involved in the killing of virus-infected macrophages by Vγ9Vδ2-T cells.10,33 Our data showed that both influenza virus-infected lung alveolar epithelial cells and PAM-expanded Vγ9Vδ2-T cells expressed high levels of Fas or FasL, and the blockade of the Fas/FasL pathway by FasL neutralizing antibody significantly inhibited the cytolytic activity of Vγ9Vδ2-T cells against influenza virus-infected cells. These findings indicate that the Fas/FasL-mediated pathway is also involved in the killing of influenza virus-infected lung alveolar epithelial cells by Vγ9Vδ2-T cells.
We confirmed that similarly to their cytotoxic function in natural killer and CD8+ T cells, perforin and granzyme B facilitated the killing of influenza virus-infected lung alveolar epithelial cells in Vγ9Vδ2-T cells. These findings are consistent with previous studies in tumors and other virus-infected cells.12,13,17 Importantly, this cytolytic response required direct cell-to-cell contact.
Of note, here, we also found that PAM-expanded Vγ9Vδ2-T cells can kill the lung alveolar epithelial cell line, A549. This is not surprising, as A549 cells are immortalized human alveolar epithelial cells and express high levels of MIC A/B, Fas, FasL, DR4 and DR5. Importantly, comparing this to A549 cells without virus infection, PAM-expanded Vγ9Vδ2-T cells had significantly higher cytotoxic activity against influenza virus-infected A549 cells. Indeed, the expression of MIC A/B and DR5 was also upregulated in A549 cells after influenza virus infection. Whether these upregulated molecules, or even some other undefined molecules, contribute to their higher cytotoxic activity against virus-infected cells will need to be determined in further studies.
In conclusion, we have demonstrated for the first time that PAM-expanded human Vγ9Vδ2-T cells can recognize and kill influenza virus-infected lung alveolar epithelial cells and thus contribute to virus clearance. The cytotoxic activity of PAM-expanded Vγ9Vδ2-T cells is dependent on cell-to-cell contact and requires NKG2D activation. The pathways of perforin–granzyme B, TRAIL and Fas–FasL are also involved in their cytotoxicity. Our study provides insight into the effector functions of Vγ9Vδ2-T cells against influenza A virus in the human body. Targeting these cells by commercially available PAM could potentially offer an alternative treatment for influenza virus infection.
References
Hu C, Qian L, Miao Y, Huang Q, Miao P, Wang P et al. Antigen-presenting effects of effector memory Vgamma9Vdelta2 T cells in rheumatoid arthritis. Cell Mol Immunol 2012; 9: 245–254.
Zhao H, Xi X, Cui L, He W . CDR3delta-grafted gamma9delta2T cells mediate effective antitumor reactivity. Cell Mol Immunol 2012; 9: 147–154.
Zheng J, Liu Y, Lau YL, Tu W . gammadelta-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol 2012; in press.
Zhou J, Kang N, Cui L, Ba D, He W . Anti-gammadelta TCR antibody-expanded gammadelta T cells: a better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol 2012; 9: 34–44.
Born WK, Reardon CL, O'Brien RL . The function of gammadelta T cells in innate immunity. Curr Opin Immunol 2006; 18: 31–38.
Carding SR, Egan PJ . Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2002; 2: 336–345.
Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D . Innate immune functions of human gammadelta T cells. Immunobiology 2008; 213: 173–182.
Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL et al. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis 2009; 200: 858–865.
Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK et al. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int Immunol 2007; 19: 657–673.
Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells 2008; 26: 562–569.
Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med 2011; 208: 1511–1522.
Bukowski JF, Morita CT, Brenner MB . Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol 1994; 153: 5133–5140.
Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P et al. Shared reactivity of Vδ2negγδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201: 1567–1578.
Poccia F, Agrati C, Castilletti C, Bordi L, Gioia C, Horejsh D et al. Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J Infect Dis 2006; 193: 1244–1249.
Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, Malkovsky M . Antiviral reactivities of gammadelta T cells. Microbes Infect 2005; 7: 518–528.
Poccia F, Agrati C, Martini F, Mejia G, Wallace M, Malkovsky M . Vgamma9Vdelta2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol Lett 2005; 100: 14–20.
Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma 9V delta 2 T lymphocytes. J Immunol 1997; 159: 6009–6017.
Sciammas R, Bluestone JA . TCRgammadelta cells and viruses. Microbes Infect 1999; 1: 203–212.
Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 2002; 360: 1831–1837.
Qin G, Liu Y, Zheng J, Ng IH, Xiang Z, Lam KT et al. Type 1 responses of human Vgamma9Vdelta2 T cells to influenza A viruses. J Virol 2011; 85: 10109–10116.
Qin G, Liu Y, Zheng J, Xiang Z, Ng IH, Malik Peiris JS et al. Phenotypic and functional characterization of human gammadelta T-cell subsets in response to influenza A viruses. J Infect Dis 2012; 205: 1646–1653.
Boullier S, Poquet Y, Halary F, Bonneville M, Fournie JJ, Gougeon ML . Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2A class I receptor on CD94− peripheral Vgamma9 Vdelta2 T cells but not on CD94− thymic or mature gammadelta T cell clones. Eur J Immunol 1998; 28: 3399–3410.
Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol 2010; 84: 6527–6535.
Zeine R, Pon R, Ladiwala U, Antel JP, Filion LG, Freedman MS . Mechanism of gammadelta T cell-induced human oligodendrocyte cytotoxicity: relevance to multiple sclerosis. J Neuroimmunol 1998; 87: 49–61.
Agrati C, Alonzi T, de Santis R, Castilletti C, Abbate I, Capobianchi MR et al. Activation of Vgamma9Vdelta2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int Immunol 2006; 18: 11–18.
Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR et al. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 2008; 14: 4232–4240.
Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C . NKG2D ligands: key targets of the immune response. Trends Immunol 2008; 29: 397–403.
Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 1997; 7: 831–836.
Fujishima N, Hirokawa M, Fujishima M, Yamashita J, Saitoh H, Ichikawa Y et al. Skewed T cell receptor repertoire of Vdelta1+ gammadelta T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein–Barr virus-infected B cells in clonal restriction. Clin Exp Immunol 2007; 149: 70–79.
Voss D, Pfefferle S, Drosten C, Stevermann L, Traggiai E, Lanzavecchia A et al. Studies on membrane topology, N-glycosylation and functionality of SARS-CoV membrane protein. Virol J 2009; 6: 79.
Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 2001; 15: 83–93.
Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T . Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol 2005; 175: 2144–2151.
Dalton JE, Howell G, Pearson J, Scott P, Carding SR . Fas–Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol 2004; 173: 3660–3667.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (No. 30973235), Science and Technology Project of the Sichuan Science and Technology Department (2010SZ0110), General Research Fund, Research Grants Council of Hong Kong (HKU 781211M) and the Area of Excellence Scheme of the University Grants Committee, Hong Kong SAR, China (AoE/M-12/06).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, H., Xiang, Z., Feng, T. et al. Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell Mol Immunol 10, 159–164 (2013). https://doi.org/10.1038/cmi.2012.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.70
Keywords
This article is cited by
-
Double-edged sword: γδ T cells in mucosal homeostasis and disease
Experimental & Molecular Medicine (2023)
-
Patients with gastrointestinal irritability after TGN1412-induced cytokine storm displayed selective expansion of gut-homing αβ and γδT cells
Cancer Immunology, Immunotherapy (2021)
-
The dark side of the spoon - glucose, ketones and COVID-19: a possible role for ketogenic diet?
Journal of Translational Medicine (2020)
-
Role of γδ T cells in controlling viral infections with a focus on influenza virus: implications for designing novel therapeutic approaches
Virology Journal (2020)
-
CD137 costimulation enhances the antiviral activity of Vγ9Vδ2-T cells against influenza virus
Signal Transduction and Targeted Therapy (2020)