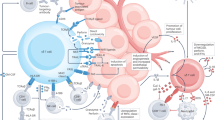
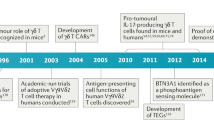
Abstract
During the last several years, research has produced a significant amount of knowledge concerning the characteristics of human γδ T lymphocytes. Findings regarding the immune functions of these cells, particularly their natural killer cell-like lytic activity against tumor cells, have raised expectations for the therapeutic applications of these cells for cancer. Pharmaceutical companies have produced selective agonists for these lymphocytes, and several teams have launched clinical trials of γδ T cell-based cancer therapies. The findings from these studies include hematological malignancies (follicular lymphoma, multiple myeloma, acute and chronic myeloid leukemia), as well as solid tumors (renal cell, breast and prostate carcinomas), consisting of samples from more than 250 patients from Europe, Japan and the United States. The results of these pioneering studies are now available, and this short review summarizes the lessons learned and the role of γδ T cell-based strategies in the current landscape of cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Meraviglia S, Caccamo N, Guggino G, Tolomeo M, Siragusa S, Stassi G et al. Optimizing tumor-reactive γδ T cells for antibody-based cancer immunotherapy. Curr Mol Med 2010; 10: 719–726.
Kabelitz D . Human γδ T lymphocytes for immunotherapeutic strategies against cancer. F1000 Med Rep 2010; 2: 45.
Kunzmann V, Wilhelm M . Adjuvant zoledronic acid for breast cancer: mechanism of action? Lancet Oncol 2011; 12: 991–992.
Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L . Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol 2012; 33: 199–206.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T . Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A 1999; 96: 6879–6884.
Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E et al. Vγ9Vδ2T cell response to colon carcinoma cells. J Immunol 2005; 175: 5481–5488.
Viey E, Laplace C, Escudier B . Peripheral γδ T-lymphocytes as an innovative tool in immunotherapy for metastatic renal cell carcinoma. Expert Rev Anticancer Ther 2005; 5: 973–986.
Dechanet J, Merville P, Berge F, Bone-Mane G, Taupin JL, Michel P et al. Major expansion of γδ T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis 1999; 179: 1–8.
Viey E, Lucas C, Romagne F, Escudier B, Chouaib S, Caignard A . Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vγ9Vδ2 T cells from renal cell carcinoma patients. J Immunother 2008; 31: 313–323.
Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC et al. Questionable relevance of γδ T lymphocytes in renal cell carcinoma. J Immunol 2008; 180: 3578–3584.
Zheng BJ, Ng SP, Chua DT, Sham JS, Kwong DL, Lam CK et al. Peripheral γδ T-cell deficit in nasopharyngeal carcinoma. Int J Cancer 2002; 99: 213–217.
Zheng BJ, Chan KW, Im S, Chua D, Sham JS, Tin PC et al. Anti-tumor effects of human peripheral γδ T cells in a mouse tumor model. Int J Cancer 2001; 92: 421–425.
Capietto AH, Martinet L, Fournie JJ . Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol 2011; 187: 1031–1038.
Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N et al. Intravesical administration of γδ T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother 2009; 58: 493–502.
Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009; 113: 4875–4884.
Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K et al. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 2011; 105: 778–786.
Bialasiewicz AA, Ma JX, Richard G . α/β− and γ/δ TCR+ lymphocyte infiltration in necrotising choroidal melanomas. Br J Ophthalmol 1999; 83: 1069–1073.
Devaud C, Bilhere E, Loizon S, Pitard V, Behr C, Moreau J et al. Antitumor activity of γδ T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res 2009; 69: 3971–3978.
Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF . Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity 2007; 27: 334–348.
Ke Y, Kapp LM, Kapp JA . Inhibition of tumor rejection by γδ T cells and IL-10. Cell Immunol 2003; 221: 107–114.
Sicard H, Al Saati T, Delsol G, Fournie JJ . Synthetic phosphoantigens enhance human Vγ9Vδ2 T lymphocytes killing of non-Hodgkin's B lymphoma. Mol Med 2001; 7: 711–722.
Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A et al. Human Vγ9Vδ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol 2012; 188: 4701–4708.
D'Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M et al. Vγ9Vδ2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol 2010; 184: 3260–3268.
Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M . Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000; 96: 384–392.
Burjanadze M, Condomines M, Reme T, Quittet P, Latry P, Lugagne C et al. In vitro expansion of γδ T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. Br J Haematol 2007; 139: 206–216.
Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S et al. Characterization and immunotherapeutic potential of γδ T-cells in patients with glioblastoma. Neuro Oncol 2009; 11: 357–367.
Lamb LS Jr . γδ T cells as immune effectors against high-grade gliomas. Immunol Res 2009; 45: 85–95.
Kabelitz D, Wesch D, Pitters E, Zoller M . Potential of human γδ T lymphocytes for immunotherapy of cancer. Int J Cancer 2004; 112: 727–732.
Bonneville M, O'Brien RL, Born WK . γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478.
Belmant C, Decise D, Fournie JJ . Phosphoantigens and aminobisphosphonates: New leads targeting γδ T lymphocytes for cancer immunotherapy. Drug Discov Today 2006; 3: 17–23.
Thompson K, Rogers MJ . Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. J Bone Miner Res 2004; 19: 278–288.
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102: 200–206.
Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F et al. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003; 102: 2310–2311.
Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G et al. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother 2011; 60: 1447–1460.
Naoe M, Ogawa Y, Takeshita K, Morita J, Shichijo T, Fuji K et al. Zoledronate stimulates γδ T cells in prostate cancer patients. Oncol Res 2010; 18: 493–501.
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 2007; 67: 7450–7457.
Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010; 161: 290–297.
Bennouna J, Levy V, Sicard H, Senellart H, Audrain M, Hiret S et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vγ9Vδ2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother 2010; 59: 1521–1530.
Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2008; 57: 1599–1609.
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T et al. Safety profile and anti-tumor effects of adoptive immunotherapy using γ-δ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 2007; 56: 469–476.
Kobayashi H, Tanaka Y, Nakazawa H, Yagi J, Minato N, Tanabe K . A new indicator of favorable prognosis in locally advanced renal cell carcinomas: γδ T-cells in peripheral blood. Anticancer Res 2011; 31: 1027–1031.
Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S et al. Zoledronate facilitates large-scale ex vivo expansion of functional γδ T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008; 10: 842–856.
Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T et al. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol 2009; 37: 956–968.
Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011; 13: 92–97.
Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδ T cells. Eur J Cardiothorac Surg 2010; 37: 1191–1197.
Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M et al. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol 2005; 175: 5471–5480.
Kunzmann V, Kimmel B, Herrmann T, Einsele H, Wilhelm M . Inhibition of phosphoantigen-mediated γδ T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology 2009; 126: 256–267.
Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A et al. Cutting edge: TGF-beta1 and IL-15 induce FOXP3+ γδ regulatory T cells in the presence of antigen stimulation. J Immunol 2009; 183: 3574–3577.
Gong G, Shao L, Wang Y, Chen CY, Huang D, Yao S et al. Phosphoantigen-activated Vγ2Vδ2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+ T regulatory cells in mycobacterial infection. Blood 2009; 113: 837–845.
Capietto AH, Martinet L, Cendron D, Fruchon S, Pont F, Fournie JJ . Phosphoantigens overcome human TCRVγ9+ γδ Cell immunosuppression by TGF-β: relevance for cancer immunotherapy. J Immunol 2010; 184: 6680–6687.
Salot S, Bercegeay S, Dreno B, Saiagh S, Scaglione V, Bonnafous C et al. Large scale expansion of Vγ9Vδ2 T lymphocytes from human peripheral blood mononuclear cells after a positive selection using MACS ‘TCR γ/δ+ T cell isolation kit’. J Immunol Methods 2009; 347: 12–18.
Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F et al. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct γδ and alphabeta T cell responses in primates. Eur J Immunol 2007; 37: 549–565.
Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother 2012; 35: 205–213.
Pont F, Familiades J, Dejean S, Fruchon S, Cendron D, Poupot M et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of alphabeta T-cell and NK-cell signatures. Eur J Immunol 2012; 42: 228–240.
Martinet L, Poupot R, Fournie JJ . Pitfalls on the roadmap to γδ T cell-based cancer immunotherapies. Immunol Lett 2009; 124: 1–8.
Capietto AH, Martinet L, Fournie JJ . How tumors might withstand γδ T-cell attack. Cell Mol Life Sci 2011; 68: 2433–2442.
Martinet L, Jean C, Dietrich G, Fournie JJ, Poupot R . PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol 2010; 80: 838–845.
Martinet L, Fleury-Cappellesso S, Gadelorge M, Dietrich G, Bourin P, Fournie JJ et al. A regulatory cross-talk between Vγ9Vδ2 T lymphocytes and mesenchymal stem cells. Eur J Immunol 2009; 39: 752–762.
Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV et al. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 2011; 20: 728–740.
Jilaveanu LB, Sznol J, Aziz SA, Duchen D, Kluger HM, Camp RL . CD70 expression patterns in renal cell carcinoma. Hum Pathol 2012; 43: 1394–9.
Diegmann J, Junker K, Loncarevic IF, Michel S, Schimmel B, von Eggeling F . Immune escape for renal cell carcinoma: CD70 mediates apoptosis in lymphocytes. Neoplasia 2006; 8: 933–938.
Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G et al. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res 2006; 66: 6816–6825.
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 2002; 99: 754–758.
Cartron G, Trappe RU, Solal-Celigny P, Hallek M . Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clin Cancer Res 2011; 17: 19–30.
Zitvogel L, Kepp O, Kroemer G . Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8: 151–160.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fournié, JJ., Sicard, H., Poupot, M. et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials?. Cell Mol Immunol 10, 35–41 (2013). https://doi.org/10.1038/cmi.2012.39
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.39
Keywords
This article is cited by
-
Human Vγ9Vδ2 T cell expansion and their cytotoxic responses against cholangiocarcinoma
Scientific Reports (2024)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Local γδ T cells: translating promise to practice in cancer immunotherapy
British Journal of Cancer (2023)
-
Human γδ T cells induce CD8+ T cell antitumor responses via antigen-presenting effect through HSP90-MyD88-mediated activation of JNK
Cancer Immunology, Immunotherapy (2023)
-
Visualizing γδ T cells by very late antigen-4-targeted positron emission tomography
European Journal of Nuclear Medicine and Molecular Imaging (2022)