Abstract
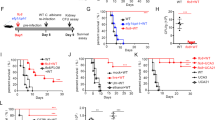
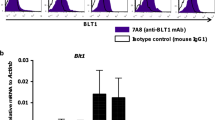
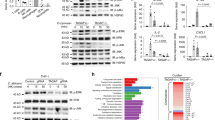
The recognition of β-glucans by dectin-1 has been shown to mediate cell activation, cytokine production and a variety of antifungal responses. Here, we report that the functional activity of dectin-1 in mucosal immunity to Candida albicans is influenced by the genetic background of the host. Dectin-1 was required for the proper control of gastrointestinal and vaginal candidiasis in C57BL/6, but not BALB/c mice; in fact, the latter showed increased resistance in the absence of dectin-1. The susceptibility of dectin-1-deficient C57BL/6 mice to infection was associated with defects in IL-17A and aryl hydrocarbon receptor-dependent IL-22 production and in adaptive Th1 responses. In contrast, the resistance of dectin-1-deficient BALB/c mice was associated with increased IL-17A and IL-22 production and the skewing towards Th1/Treg immune responses that provide immunological memory. Disparate canonical/noncanonical NF-κB signaling pathways downstream of dectin-1 were activated in the two different mouse strains. Thus, the net activity of dectin-1 in antifungal mucosal immunity is dependent on the host's genetic background, which affects both the innate cytokine production and the adaptive Th1/Th17 cell activation upon dectin-1 signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Drummond RA, Brown GD . The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011; 14: 392–399 .
Drummond RA, Brown GD . The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011; 79: 3966–3977.
Romani L . Immunity to fungal infections. Nat Rev Immunol 2011; 11: 275–288.
Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 2002; 169: 3876–3882.
Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010; 116: 5394–5402.
Lee HM, Yuk JM, Shin DM, Jo EK . Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol 2009; 29: 795–805.
Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007; 8: 39–46.
Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007; 8: 31–38.
Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 2009; 182: 4938–4946.
Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 2006; 116: 1642–1650.
Heinsbroek SE, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S . Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog 2008; 4: e1000218.
LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007; 8: 630–638.
Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 2009; 10: 203–213.
Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 2009; 361: 1760–1767.
Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis 2009; 49: 724–732.
Gales A, Conduche A, Bernad J, Lefevre L, Olagnier D, Beraud M et al. PPARgamma controls dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog 2010; 6: e1000714.
Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332: 65–68.
Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010; 32: 681–691.
Zelante T, Luca A, Romani L . TH17 Cells in Fungal Infections. In: Jiang S (ed.) TH17 Cells in Health and Disease. New York: Springer 2011: 299–317.
del Pilar Jimenez AM, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SE et al. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun 2008; 9: 338–348.
Heinsbroek SE, Taylor PR, Rosas M, Willment JA, Williams DL, Gordon S et al. Expression of functionally different dectin-1 isoforms by murine macrophages. J Immunol 2006; 176: 5513–5518.
Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D . CRZ1, a target of the calcineurin pathway in Candida albicans. Mol Microbiol 2006; 59: 1429–1451.
de Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 2010; 3: 361–373.
Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206: 299–311.
Agrawal S, Gupta S, Agrawal A . Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One 2010; 5: e13418.
Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005; 22: 507–517.
Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol 2011; 90: 357–366.
Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood 2011; 117: 6825–6836.
Autenrieth SE, Autenrieth IB . Variable antigen uptake due to different expression of the macrophage mannose receptor by dendritic cells in various inbred mouse strains. Immunology 2009; 127: 523–529.
Liu T, Matsuguchi T, Tsuboi N, Yajima T, Yoshikai Y . Differences in expression of Toll-like receptors and their reactivities in dendritic cells in BALB/c and C57BL/6 mice. Infect Immun 2002; 70: 6638–6645.
Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol 2010; 40: 2450–2459.
Stockinger B, Hirota K, Duarte J, Veldhoen M . External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol 2011; 23: 99–105.
Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW et al. Dectin-1 dependent IL-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun 2012; 80: 410–417 .
Yano J, Lilly E, Barousse M, Fidel PL Jr . Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 2010; 78: 5126–5137.
Stejskalova L, Dvorak Z, Pavek P . Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab 2011; 12: 198–212.
Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med 2011; 208: 369–381.
Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D et al. Neutrophils produce IL-17A in a Dectin-1 and IL-23 dependent manner during invasive fungal infection. Infect Immun 2011; 79: 3966–3977.
Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M . Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009; 31: 321–330.
Acknowledgements
We thank Dr Cristina Massi Benedetti for the digital art and editing. The studies were supported by the Specific Targeted Research Project ‘ALLFUN’ (FP7-HEALTH-2009 contract no. 260338 to LR) and the Italian Project AIDS 2010 by ISS (Istituto Superiore di Sanità contract no. 40H40 to LR) and Fondazione Cassa di Risparmio di Perugia Project no. 2011.0124.021. AC and CC were financially supported by fellowships from Fundação para a Ciência e Tecnologia, Portugal (contracts SFRH/BPD/46292/2008 and SFRH/BD/65962/2009, respectively).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvalho, A., Giovannini, G., De Luca, A. et al. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol 9, 276–286 (2012). https://doi.org/10.1038/cmi.2012.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.1
Keywords
This article is cited by
-
Phagosomal signalling of the C-type lectin receptor Dectin-1 is terminated by intramembrane proteolysis
Nature Communications (2022)
-
Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion
Nature Communications (2019)
-
Genetic Regulation of the Host-Fungus Interaction in the Pathogenesis of Aspergillosis
Current Fungal Infection Reports (2019)
-
The evidence for fungus in Crohn’s disease pathogenesis
Clinical Journal of Gastroenterology (2018)
-
Dexamethasone induced inhibition of Dectin-1 activation of antigen presenting cells is mediated via STAT-3 and NF-κB signaling pathways
Scientific Reports (2017)