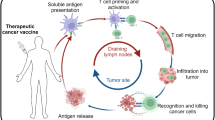
Abstract
Tumor metastases and relapse are the major causes of morbidity and mortality in cancer. Although surgery, chemotherapy and/or radiation therapy can typically control primary tumor growth, metastatic and relapsing tumors are often inaccessible or resistant to these treatments. An adaptive immune response can be generated during these conventional treatments of the primary tumor, and presumably both the primary tumor and secondary metastases share many of the same or similar antigenic characteristics recognized by the immune system. Thus, when established, this response should be able to control metastatic growth and tumor relapse. This review summarizes the mechanisms by which antitumor immune responses are generated, and recent findings supporting the hypothesis that many therapies targeting primary tumors can generate antitumor adaptive immune responses to prevent metastases and tumor relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mina LA, Sledge GW Jr . Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol 2011; 8: 325–332.
Schreiber H . Ward PL Rowley DA Stauss HJ Unique tumor-specific antigens. Annu Rev Immunol 1988; 6: 465–483.
Adler AJ . Peripheral tolerization of effector and memory T cells: implications for autoimmunity and tumor-immunity. Curr Immunol Rev 2005; 1: 21–28.
Nossal GJ . Tolerance and ways to break it. Ann NY Acad Sci 1993; 690: 34–41.
Zhou P, Fang X, McNally B, Yu P, Zhu M, Fu YX et al. Targeting lymphotoxin-mediated negative selection to prevent prostate cancer in mice with genetic predisposition. Proc Natl Acad Sci USA 2009; 106: 17134–17139.
Pardoll DM . Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol 2002; 2: 227–238.
Zou W . Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5: 263–274.
Rosenberg SA, Yang JC, Restifo NP . Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–915.
Ridgway D . The first 1000 dendritic cell vaccinees. Cancer Invest 2003; 21: 873–886.
Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T et al. Dendritic cell-based vaccination in solid cancer. J Clin Oncol 2003; 21: 135–142.
Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 1999; 190: 1669–1678.
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 2003; 198: 569–580.
Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L et al. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med 1997; 186: 229–238.
Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother 2001; 24: 363–373.
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298: 850–854.
Dougan M, Dranoff G . Immune therapy for cancer. Annu Rev Immunol 2009; 27: 83–117.
Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26: 1789–1796.
Clynes RA, Towers TL, Presta LG, Ravetch JV . Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 2000; 6: 443–446.
Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 2011; 19: 101–113.
Politi K, Pao W . How genetically engineered mouse tumor models provide insights into human cancers. J Clin Oncol 2011; 29: 2273–2281.
Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 2007; 13: 5133–5143.
Garrido G, Lorenzano P, Sánchez B, Beausoleil I, Alonso DF, Pérez R et al. T cells are crucial for the anti-metastatic effect of anti-epidermal growth factor receptor antibodies. Cancer Immunol Immunother 2007; 56: 1701–1710.
Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18: 160–170.
Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA 2011; 108: 7142–7147.
Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010; 18: 485–498.
Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J . Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res 2008; 68: 7530–7540.
Xuan C, Steward KK, Timmerman JM, Morrison SL . Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood 2010; 115: 2864–2871.
Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–7523.
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114: 589–595.
Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR . Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA 1989; 86: 10104–10107.
Zhang JS, Nakatsugawa S, Niwa O, Ju GZ, Liu SZ . Ionizing radiation-induced IL-1 alpha, IL-6 and GM-CSF production by human lung cancer cells. Chin Med J (Engl) 1994; 107: 635 657.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271.
Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW . External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004; 64: 4328–4337.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13: 1050–1059.
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 2011; 71: 2488–2496.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G . Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8: 59–73.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61.
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004; 34: 336–344.
Lutsiak ME, Semnani RT, de Pascalis R, Kashmiri SV, Schlom J, Sabzevari H . Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005; 105: 2862–2868.
Liu J, Zhao J, Hu L, Cao Y, Huang B . Low dosages: new chemotherapeutic weapons on the battlefield of immune-related disease. Cell Mol Immunol 2011; 8: 289–295.
Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005; 106: 2018–2025.
Hegde U, Chhabra A, Chattopadhyay S, Das R, Ray S, Chakraborty NG . Presence of low dose of fludarabine in cultures blocks regulatory T cell expansion and maintains tumor-specific cytotoxic T lymphocyte activity generated with peripheral blood lymphocytes. Pathobiology 2008; 75: 200–208.
Correale P, Cusi MG, Tsang KY, del Vecchio MT, Marsili S, Placa ML et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 2005; 23: 8950–8958.
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26: 5233–5239.
Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B . Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res 2010; 70: 4850–4858.
Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity 2006; 25: 499–509.
Aloisi F, Pujol-Borrell R . Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006; 6: 205–217.
Ruddle NH . Lymphoid neo-organogenesis: lymphotoxin's role in inflammation and development. Immunol Res 1999; 19: 119–125.
Fu YX, Chaplin DD . Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999; 17: 399–433.
Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K . The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 1998; 9: 59–70.
Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD . Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 1996; 271: 1289–1291.
Rennert PD, James D, Mackay F, Browning JL, Hochman PS . Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity 1998; 9: 71–79.
Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med 1999; 189: 403–412.
Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH . Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol 1998; 161: 6853–6860.
Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH . Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med 2003; 197: 1153–1163.
Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH . Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol 2000; 156: 1133–1138.
Kratz A, Campos-Neto A, Hanson MS, Ruddle NH . Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med 1996; 183: 1461–1472.
Spriggs DR, Sherman ML, Michie H, Arthur KA, Imamura K, Wilmore D et al. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase I and pharmacologic study. J Natl Cancer Inst 1988; 80: 1039–1044.
Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA et al. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity 2001; 14: 111–121.
Cyster JG . Chemokines and cell migration in secondary lymphoid organs. Science 1999; 286: 2098–2102.
Kirk CJ, Hartigan-O'Connor D, Mule JJ . The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res 2001; 61: 8794–8802.
Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 1998; 8: 21–30.
Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC et al. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem 2000; 275: 14307–14315.
Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med 2000; 6: 283–289.
Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 1998; 102: 1142–1151.
Wang J, Foster A, Chin R, Yu P, Sun Y, Wang Y et al. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol 2002; 32: 1969–1979.
Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest 2001; 108: 1771–1780.
Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004; 5: 141–149.
Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF et al. Increasing tumor antigen expression overcomes ‘ignorance’ to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 2002; 17: 737–747.
Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol 2000; 164: 4558–4563.
Wolkers MC, Brouwenstijn N, Bakker AH, Toebes M, Schumacher TN . Antigen bias in T cell cross-priming. Science 2004; 304: 1314–1317.
Chen L . Overcoming T cell ignorance by providing costimulation. Implications for the immune response against cancer. Adv Exp Med Biol 1998; 451: 159–165.
Lenschow DJ, Walunas TL, Bluestone JA . CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14: 233–258.
Bai XF, Gao JX, Liu J, Wen J, Zheng P, Liu Y . On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res 2001; 61: 6860–6867.
Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol 2007; 179: 1960–1968.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, X., Mortenson, E. & Fu, YX. Targeting and utilizing primary tumors as live vaccines: changing strategies. Cell Mol Immunol 9, 20–26 (2012). https://doi.org/10.1038/cmi.2011.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2011.49