Abstract
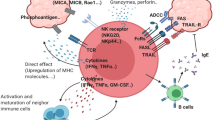
Cell-based immunotherapy for lymphoid malignancies has gained increasing attention as patients develop resistance to conventional treatments. γδ T cells, which have major histocompatibility complex (MHC)-unrestricted lytic activity, have become a promising candidate population for adoptive cell transfer therapy. We previously established a stable condition for expanding γδ T cells by using anti-γδ T-cell receptor (TCR) antibody. In this study, we found that adoptive transfer of the expanded γδ T cells to Daudi lymphoma-bearing nude mice significantly prolonged the survival time of the mice and improved their living status. We further investigated the characteristics of these antibody-expanded γδ T cells compared to the more commonly used phosphoantigen-expanded γδ T cells and evaluated the feasibility of employing them in the treatment of lymphoid malignancies. Slow but sustained proliferation of human peripheral blood γδ T cells was observed upon stimulation with anti-γδ TCR antibody. Compared to phosphoantigen-stimulated γδ T cells, the antibody-expanded cells manifested similar functional phenotypes and cytotoxic activity towards lymphoma cell lines. It is noteworthy that the anti-γδ TCR antibody could expand both the Vδ1 and Vδ2 subsets of γδ T cells. The in vitro-expanded Vδ1 T cells displayed comparable tumour cell-killing activity to Vδ2 T cells. Importantly, owing to higher C–C chemokine receptor 4 (CCR4) and CCR8 expression, the Vδ1 T cells were more prone to infiltrate CCL17- or CCL22-expressing lymphomas than the Vδ2 T cells. Characterizing the peripheral blood γδ T cells from lymphoma patients further confirmed that the anti-γδ TCR antibody-expanded γδ T cells could be a more efficacious choice for the treatment of lymphoid malignancies than phosphoantigen-expanded γδ T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Long HM, Parsonage G, Fox CP, Lee SP . Immunotherapy for Epstein–Barr virus-associated malignancies. Drug News Perspect 2010; 23: 221–228.
Dunn GP, Old LJ, Schreiber RD . The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21: 137–148.
Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N . Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol 2001; 167: 5092–5098.
Bonneville M, O'Brien RL, Born WK . Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478.
Kunzmann V, Bauer E, Wilhelm M . γ/δ T-cell stimulation by pamidronate. N Engl J Med 1999; 340: 737–738.
Bennouna J, Levy V, Sicard H, Senellart H, Audrain M, Hiret S et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother 2010; 59: 1521–1530.
Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010; 161: 290–297.
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T et al. Safety profile and antitumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 2007; 56: 469–476.
Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011; 13: 92–97.
Sakamoto M, Nakajima J, Murakawa T, Fukami T, Yoshida Y, Murayama T et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδT cells: a phase I clinical study. J Immunother 2011; 34: 202–211.
Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K . Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res 2010; 30: 575–579.
Qi J, Zhang J, Zhang S, Cui L, He W . Immobilized MICA could expand human Vdelta1 gammadelta T cells in vitro that displayed major histocompatibility complex class I chain-related A-dependent cytotoxicity to human epithelial carcinomas. Scand J Immunol 2003; 58: 211–220.
Kabelitz D, Wesch D, He W . Perspectives of gammadelta T cells in tumor immunology Cancer Res 2007; 67: 5–8.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T . Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MIC-A and MIC-B. Proc Natl Acad Sci USA 1999; 96: 6879–6884.
Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M et al. Vδ1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res 2004; 64: 9172–9179.
Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y et al. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther 2009; 8: 1540–1549.
Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med 1996; 183: 1681–1696.
Sireci G, Espinosa E, Di Sano C, Dieli F, Fournié JJ, Salerno A . Differential activation of human gammadelta cells by nonpeptide phosphoantigens. Eur J Immunol 2001; 31: 1628–1635.
Zhao J, Huang J, Chen H, Cui L, He W . Vdelta1 T cell receptor binds specifically to MHC I chain related A: molecular and biochemical evidences. Biochem Biophys Res Commun 2006; 339: 232–240.
Cao W, Xi X, Wang Z, Dong L, Hao Z, Cui L et al. Four novel ULBP splice variants are ligands for human NKG2D. Int Immunol 2008; 20: 981–991.
Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A–I. Immunity 2005; 22: 71–80.
Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE et al. Human γδ T lymphocytes induce robust NK cell mediated antitumor cytotoxicity through CD137 engagement. Blood 2010; 116: 1726–1733.
Shrestha N, Ida JA, Lubinski AS, Pallin M, Kaplan G, Haslett PA . Regulation of acquired immunity by gamma delta T-cell/dendritic-cell interactions. Ann NY Acad Sci 2005; 1062: 79–94.
Brandes M, Willimann K, Bioley G, Lévy N, Eberl M, Luo M et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA 2009; 106: 2307–2312.
Lozupone F, Pende D, Burgio VL, Castelli C, Spada M, Venditti M et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res 2004; 64: 378–385.
Kabelitz D, Wesch D, Pitters E, Zöller M . Characterization of tumor reactivity of human Vgamma9Vdelta2 gammadelta T cells in vitro and in SCID mice in vivo. J Immunol 2004; 173: 6767–6776.
Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, de Libero G . Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197: 163–168.
Ikeda H, Old LJ, Schreiber RD . The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 2002; 13: 95–109.
Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G et al. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood 2004; 104: 1801–1807.
Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med 2003; 198: 391–397.
Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N . Involvement of CD166 in the activation of human gamma delta T cells by tumor cells sensitized with nonpeptide antigens. J Immunol 2006; 177: 877–884.
Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood 2008; 111: 2339–2346.
Aruffo A, Melnick MB, Linsley PS, Seed B . The lymphocyte glycoprotein CD6 contains a repeated domain structure characteristic of a new family of cell surface and secreted proteins. J Exp Med 1991; 174: 949–952.
Aruffo A, Bowen MA, Patel DD, Haynes BF, Starling GC, Gebe JA et al. CD6-ligand interactions: a paradigm for SRCR domain function? Immunol Today 1997; 18: 498–504.
Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood 2010; 115: 2407–2411.
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8: 765–772.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB et al. Tumor associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800.
Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M et al. PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008; 111: 3220–3224.
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65: 1089–1096.
Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood 2007; 109: 2078–2085.
Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011; 11: in press.
Dokouhaki P, Han M, Joe B, Li M, Johnston MR, Tsao MS et al. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: a new approach. Cancer Lett 2010; 297: 126–136.
Nakanishi T, Imaizumi K, Hasegawa Y, Kawabe T, Hashimoto N, Okamoto M et al. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol Immunother 2006; 55: 1320–1329.
Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 2008; 122: 2286–2293.
Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM . Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 2006; 107: 3639–3646.
Peh SC, Kim LH, Poppema S . TARC, a CC chemokine, is frequently expressed in classic Hodgkin's lymphoma but not in NLP Hodgkin's lymphoma, T-cell-rich B-cell lymphoma, and most cases of anaplastic large cell lymphoma. Am J Surg Pathol 2001; 25: 925–929.
Thielen C, Radermacher V, Trimeche M, Roufosse F, Goldman M, Boniver J et al. TARC and IL-5 expression correlates with tissue eosinophilia in peripheral T-cell lymphomas. Leuk Res 2008; 32: 1431–1438.
van den Berg A, Visser L, Poppema S . High expression of the CC chemokine TARC in Reed–Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. Am J Pathol 1999; 154: 1685–1691.
Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S . Chemokines, cytokines and their receptors in Hodgkin's lymphoma cell lines and tissues. Ann Oncol 2002; 13 ( Suppl 1): 52–56.
Acknowledgements
This work was supported by two grants (No. 2006AA02Z480 and No. 2007AA021109) from the National High Technology Research and Development Program (863 Program), and one grant (No. 30972776) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, J., Kang, N., Cui, L. et al. Anti-γδ TCR antibody-expanded γδ T cells: a better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol Immunol 9, 34–44 (2012). https://doi.org/10.1038/cmi.2011.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2011.16
Keywords
This article is cited by
-
miR-125b-5p and miR-99a-5p downregulate human γδ T-cell activation and cytotoxicity
Cellular & Molecular Immunology (2019)
-
Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation
Journal for ImmunoTherapy of Cancer (2017)
-
The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy
Cellular & Molecular Immunology (2015)
-
Distortion of memory Vδ2 γδ T cells contributes to immune dysfunction in chronic HIV infection
Cellular & Molecular Immunology (2015)
-
Cytotoxic and Regulatory Properties of Circulating Vδ1+ γδ T Cells: A New Player on the Cell Therapy Field?
Molecular Therapy (2014)