Abstract
A hybrid vector of adeno-associated virus and phage (termed AAVP) has been introduced as a platform for systemic ligand-directed delivery of transgenes to tumors over the past decade. A series of studies have evaluated the AAVP platform for potential theranostic or purely therapeutic applications in several tumor models. Sufficient ligand-directed tumor targeting consistently resulted in specific molecular-genetic imaging and/or anti-tumor responses to ‘suicide’ transgene delivery. However, efforts to optimize transduction efficiency are still ongoing. Here, we set out to expand the translational utility of AAVP by combining it with gold (Au) nanoparticles in order to generate a ‘transducing matrix’ for improved targeted gene delivery in solid phase. Targeted AAVP-based solid-phase transduction is superior to conventional transduction in soluble (aqueous) environments. This transducing matrix is stable and can be further modified with additional attributes (for example, magnetization) for targeted imaging and therapeutic gene delivery. Notably, it spontaneously assembles around cells in vitro to markedly enhance transduction capabilities compared with AAVP alone. This versatile nanoplatform may enable new applications of AAVP for transgene delivery in translational settings including, for example, efforts toward complex tissue patterning.
Similar content being viewed by others
Introduction
The pIII coat protein of bacteriophage (phage) can be modified to display peptide ligands that are selected to home to a corresponding target receptor on a desired cell population. Such a targeting attribute has great potential for clinical applications including gene therapy approaches, particularly against cancer. Indeed, in the decade since phage was first combined with AAV to yield AAVP (adeno-associated virus and phage),1 which has a high transduction efficiency for human cells, numerous constructs have been tested for theranostic and therapeutic applications in preclinical cancer models.2, 3, 4, 5, 6, 7, 8 Phage particles have also been more recently combined with gold (Au) nanoparticles to generate a nanoscaffold that self-assembles spontaneously as a result of the physical and biological properties of its various components.9, 10 These aggregates are stable fractal networks with high surface area, formed from electrostatic interactions between amino acids on the phage pVIII capsid proteins and the dispersed Au nanoparticles and subject to fluctuations in phage input, pH and salt concentration, which, when optimized, can minimize Au agglomeration and maximize network stability.9, 10 The properties of these peptide-targeted scaffolds can be further tuned for translational applications by introducing various functional nanoparticles, including additional elements such as imidazole to adjust the optical properties,9 iron oxide to generate magnetic fields11 and liposomes loaded with therapeutic or imaging cargo.12 Here, we hypothesized that including AAVP (as opposed to phage particles alone) in these nanoscaffolds would generate a stable, modifiable matrix that would transduce mammalian cells with higher efficiency and thereby improve transgene delivery in several settings.
Results
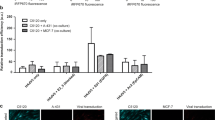
As a proof-of-concept, we evaluated the efficacy of the self-assembled transducing matrix in cells growing in tissue culture. First, an AAVP displaying the double-cyclic RGD-4C ligand motif (sequence CDCRGDCFC) and carrying a green fluorescent protein-encoding transgene under the control of a standard CMV promoter13 served to form the nanoscaffold and was administered to cells in culture (Figure 1). The nanoscaffold was formed as described9, 10, 11 but with increasing amounts of AAVP particles substituted for phage particles. In our academic and commercial pipeline, after common citrate reduction using Au(III) chloride (⩾99.99%, Sigma-Aldrich, St. Louis, MO, USA), Au nanoparticles used in scaffold formation are consistently 45–50 nm.9, 10, 11 Although RGD-4C phage is ~6 nm in diameter and just under 1 μm in length, the inclusion of an AAV genomic cassette into the phage genome appears to increase the resulting hybrid particle length,9, 14 with no detectable differences in scaffold properties. At all amounts measured and conditions evaluated, the transduction efficiency was substantially higher by using the transducing matrix relative to AAVP alone. We have previously demonstrated that incubating cells with an Au-phage scaffold result in strong accumulation of a molecular network in the cells that remains even after washings.9 This empiric biologic phenomenon likely leads to retention in and around the cells, resulting in a greater percent of transduced cells by using the AAVP-based transducing matrix compared to AAVP alone. Additionally, previous work has shown that the combination of Au nanoparticles and RGD-4C phage particles produces an aggregate network that is relatively ‘loose,’ with a lower fractal dimension than more compact imidazole-containing constructs, exposing binding sites and producing a distinct kinetic profile that results in cellular internalization.9 This increased internalization efficiency compared to other scaffold formulations are retained here, and such receptor-mediated cell internalization is critical for targeted AAVP transduction. Although these experimental conditions may certainly be further optimized, the proof-of-concept studies presented here reveal a novel nanoengineering platform for biological investigation and development into translational applications.
Comparison of adeno-associated virus and phage (AAVP) alone and AAVP and gold (Au) nanoparticles in the transducing matrix in solid phase. (a) Green fluorescent protein (GFP) expression (green) in KS1767 Kaposi sarcoma cells incubated with targeted or insertless control AAVP alone or the corresponding transducing matrices. (b) Relative quantification of transduced cells after incubation with either targeted or insertless (negative control) AAVP alone or in a transducing matrix construct.
Discussion
Recently, a phage-based scaffold was evaluated for its translational potential in several animal models.12 Targeting activity was confirmed in vivo, revealing tumor homing and scaffold aggregation prior to cargo delivery, and, because components of the networks can be imaged independently, this scaffold should provide a unique, biocompatible tool for enhanced gene expression. In addition to the utility of this nanoscaffold for peptide-guided therapeutic gene delivery and reporter gene imaging, the integration of additional biocompatible nanoparticles, such as silicon (Si) or iron (FeO),11 into the transducing matrix could yield further practical applications including signal reporters and/or temperature sensors. Optimizing AAVP input in relation to transduction efficiency during preclinical studies could yield many translational implications. Moreover, because of its greater transduction efficiency in vitro compared to AAVP alone, the utility of the transducing matrix as a screening tool to validate ligands and identify susceptible cell lines for future studies should become evident. Indeed, incorporation of alternate nanoparticles for gene transduction in solid phase would likely provide a uniquely engineered platform for imaging (fluorescence, darkfield or magnetic resonance imaging) or other signal reporting (surface-enhanced Raman scattering, SERS), and even tunable photon-to-heat conversion.9, 12, 15 Finally, theranostic applications for AAVP are being investigated more frequently now that the technology has matured.7, 8 We have already begun optimizing the vector itself for specific tumor types and their corresponding molecular markers. By incorporating tumor-specific promoters for transcriptional targeting8 and rationally chosen motifs for ligand–receptor-directed targeting,4 we have moved AAVP from a preclinical tool toward an enabling solid-phase platform with tantalizing translational ramifications. Specifically, this initial brief report outlines the critical optimization of transduction efficiency in solid phase with demonstrably superior efficacy compared to AAVP alone, potentially increasing clinical utility, although simultaneously enabling tumor trafficking and accumulation to be monitored, which is useful for a repetitive dosing schedule, because Au nanoparticles are biocompatible and distinguishable by X-ray-based imaging including computed tomography. Although one type of AAVP vectors contains a theranostic reporter transgene suitable for both imaging and therapy after administration of an appropriate substrate,1, 2, 5, 7, 8 a second type of AAVP is being pursued with a cytokine transgene not amenable to imaging,3, 4, 6 which could be directly imaged by using a transducing matrix. In vivo studies will be critical for evaluating these translational transducing matrix properties. Further arguments could be made that the transducing matrix is particularly suitable for tissue engineering in which multiple types of cells would have to be transduced in a pattern to form complex three-dimensional matrices. Rather than direct immobilization of viral particles alone to encourage transduction, the inclusion of magnetic particles would restrict accessibility to cells in specific patterns or spatial confines, while receptor expression profiles enable molecularly permissive transduction of ligand-directed particles. Within the context of the first decade and beyond from the introduction of targeted AAVP as a viable man-made mammalian cell-transducing viral particle, the new results introduced here further highlight some of the potential of this technology at large.
References
Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC 3rd et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006; 125: 385–398.
Hajitou A, Lev DC, Hannay JA, Korchin B, Staquicini FI, Soghomonyan S et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc Natl Acad Sci USA 2008; 105: 4471–4476.
Paoloni MC, Tandle A, Mazcko C, Hanna E, Kachala S, Leblanc A et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS ONE 2009; 4: e4972.
Smith TL, Yuan Z, Cardó-Vila M, Sanchez Claros C, Adem A, Cui MH et al. AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proc Natl Acad Sci USA 2016; 113: 2466–2471.
Staquicini FI, Ozawa MG, Moya CA, Driessen WH, Barbu EM, Nishimori H et al. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastoma. J Clin Invest 2011; 121: 161–173.
Tandle A, Hanna E, Lorang D, Hajitou A, Moya CA, Pasqualini R et al. Tumor vasculature-targeted delivery of tumor necrosis factor-alpha. Cancer 2009; 115: 128–139.
Ferrara F, Staquicini DI, Driessen WHP, D'Angelo S, Dobroff AS, Barry M et al. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proc Natl Acad Sci USA 2016; 113: 12786–12791.
Dobroff AS, D'Angelo S, Eckhardt BL, Ferrara F, Staquicini DI, Cardo-Vila M et al. Towards a transcriptome-based theranostic platform for unfavorable breast cancer phenotypes. Proc Natl Acad Sci USA 2016; 113: 12780–12785.
Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL et al. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Natl Acad Sci USA 2006; 103: 1215–1220.
Souza GR, Yonel-Gumruk E, Fan D, Easley J, Rangel R, Guzman-Rojas L et al. Bottom-up assembly of hydrogels from bacteriophage and Au nanoparticles: the effect of cis- and trans-acting factors. PLoS ONE 2008; 3: e2242.
Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol 2010; 5: 291–296.
Hosoya H, Dobroff AS, Driessen WH, Cristini V, Brinker LM, Staquicini FI et al. Integrated nanotechnology platform for tumor-targeted multimodal imaging and therapeutic cargo release. Proc Natl Acad Sci USA 2016; 113: 1877–1882.
Hajitou A, Rangel R, Trepel M, Soghomonyan S, Gelovani JG, Alauddin MM et al. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat Protoc 2007; 2: 523–531.
Stoneham CA, Hollinshead M, Hajitou A . Clathrin-mediated endocytosis and subsequent endo-lysosomal trafficking of adeno-associated virus/phage. J Biol Chem 2012; 287: 35849–35859.
Souza GR, Levin CS, Hajitou A, Pasqualini R, Arap W, Miller JH . In vivo detection of gold-imidazole self-assembly complexes: NIR-SERS signal reporters. Anal Chem 2006; 78: 6232–6237.
Acknowledgements
We thank Dr Helen Pickersgill (Life Science Editors) for critical reading and editing of the manuscript. This work was supported in part by an award from the Gillson Longenbaugh Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
GRS serves as President and Chief Scientific Officer and has equity in Nano3D Biosciences. WA and RP are founders of AAVP BioSystems, which has licensed intellectual property related to the adeno-associated virus/phage (AAVP) technology, and they are inventors on patent applications and entitled to standard royalties if commercialization occurs. The University of New Mexico Health Sciences Center currently manages these arrangements in accordance with its established institutional conflict of interest policy. The remaining authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Smith, T., Souza, G., Sidman, R. et al. An AAVP-based solid-phase transducing matrix for transgene delivery: potential for translational applications. Cancer Gene Ther 24, 358–360 (2017). https://doi.org/10.1038/cgt.2017.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2017.19