Abstract
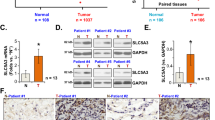
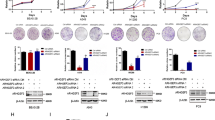
The aim of this research is to determine the role of human asparagine synthetase (ASNS) in human lung cancer. In the present study, immunohistochemical staining and the Oncomine database mining showed that the expression of ASNS gene was higher in lung cancer tissues than that in the normal tissues by. In addition, western blot assay showed that ASNS was elevated in lung cancer A549 and 95D cell lines as compared with that in H1299 and H460 cells. Therefore, A549 and 95D cells were chosen for subsequent MTT and colony formation assay. It was found that knockdown of ASNS inhibited the growth and colony formation abilities of A549 and 95D cells. Flow cytometry showed that ASNS silencing arrested cell cycle progression at G0/G1 phase in A549 cells, probably through regulating the expression of cell cycle molecules such as CDK2 and Cyclin E1 as shown by quantitative real-time PCR. Taken together, our study indicates that ASNS may be an important target for lung cancer diagnosis and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132.
Richards NG, Kilberg MS . Asparagine synthetase chemotherapy. Annu Rev Biochem 2006; 75: 629–654.
Kilberg MS, Barbosa-Tessmann IP . Genomic sequences necessary for transcriptional activation by amino acid deprivation of mammalian cells. J Nutr 2002; 132: 1801–1804.
Barbosa-Tessmann IP, Pineda VL, Nick HS, Schuster SM, Kilberg MS . Transcriptional regulation of the human asparagine synthetase gene by carbohydrate availability. Biochem J 1999; 339(Pt 1):151–158.
Hutson RG, Kitoh T, Moraga Amador DA, Cosic S, Schuster SM, Kilberg MS . Amino acid control of asparagine synthetase: relation to asparaginase resistance in human leukemia cells. Am J Physiol 1997; 272: C1691–C1699.
Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M et al. Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin. Cancer Res 2007; 67: 3345–3355.
Lorenzi PL, Llamas J, Gunsior M, Ozbun L, Reinhold WC, Varma S et al. Asparagine synthetase is a predictive biomarker of l-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther 2008; 7: 3123–3128.
Lorenzi PL, Reinhold WC, Rudelius M, Gunsior M, Shankavaram U, Bussey KJ et al. Asparagine synthetase as a causal, predictive biomarker for L-asparaginase activity in ovarian cancer cells. Mol Cancer Ther 2006; 5: 2613–2623.
Zhang B, Dong LW, Tan YX, Zhang J, Pan YF, Yang C et al. Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. Br J Cancer 2013; 109: 14–23.
Sircar K, Huang H, Hu L, Cogdell D, Dhillon J, Tzelepi V et al. Integrative molecular profiling reveals asparagine synthetase is a target in castration-resistant prostate cancer. Am J Pathol 2012; 180: 895–903.
Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002; 8: 816–824.
Hou J, Aerts J, den Hamer B, van Ijcken W, den Bakker M, Riegman P et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS ONE 2010; 5: e10312.
Selamat SA, Chung BS, Girard L, Zhang W, Zhang Y, Campan M et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res 2012; 22: 1197–1211.
Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA Jr et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol 2005; 167: 1763–1775.
Wang J, Yu S, Cui L, Wang W, Li J, Wang K et al. Role of SMC1A overexpression as a predictor of poor prognosis in late stage colorectal cancer. BMC Cancer 2015; 15: 90.
Zhou W, Wang Z, Shen N, Pi W, Jiang W, Huang J et al. Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol Cell Biochem 2015; 398: 11–19.
Kilberg MS, Pan YX, Chen H, Leung-Pineda V . Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 2005; 25: 59–85.
Colletta G, Cirafici AM . TSH is able to induce cell cycle-related gene expression in rat thyroid cell. Biochem Biophys Res Commun 1992; 183: 265–272.
Greco A, Ittmann M, Basilico C . Molecular cloning of a gene that is necessary for G1 progression in mammalian cells. Proc Natl Acad Sci USA 1987; 84: 1565–1569.
Hongo S, Takeda M, Sato T . Induction of asparagine synthetase during lymphocyte activation by phytohemagglutinin. Biochem Int 1989; 18: 661–666.
Verma N, Kumar K, Kaur G, Anand S . l-asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol 2007; 27: 45–62.
Appel IM, den Boer ML, Meijerink JP, Veerman AJ, Reniers NC, Pieters R . Up-regulation of asparagine synthetase expression is not linked to the clinical response L-asparaginase in pediatric acute lymphoblastic leukemia. Blood 2006; 107: 4244–4249.
Dufour E, Gay F, Aguera K, Scoazec JY, Horand F, Lorenzi PL et al. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas 2012; 41: 940–948.
Li H, Zhou F, Du W, Dou J, Xu Y, Gao W et al. Knockdown of asparagine synthetase by RNAi suppresses cell growth in human melanoma cells and epidermoid carcinoma cells. Biotechnol Appl Biochem 2015; 63: 328–333.
Gong SS, Basilico C . A mammalian temperature-sensitive mutation affecting G1 progression results from a single amino acid substitution in asparagine synthetase. Nucleic Acids Res 1990; 18: 3509–3513.
Morgan DO . Principles of CDK regulation. Nature 1995; 374: 131–134.
Weinberg RA . The retinoblastoma protein and cell cycle control. Cell 1995; 81: 323–330.
Ekholm SV, Reed SI . Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol 2000; 12: 676–684.
Sherr CJ . G1 phase progression: cycling on cue. Cell 1994; 79: 551–555.
Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S et al. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 1992; 257: 1689–1694.
Malumbres M, Barbacid M . Cell cycle kinases in cancer. Curr Opin Genet Dev 2007; 17: 60–65.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81572246).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, Y., Lv, F., Zhu, X. et al. Loss of asparagine synthetase suppresses the growth of human lung cancer cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther 23, 287–294 (2016). https://doi.org/10.1038/cgt.2016.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2016.28
This article is cited by
-
Targeting TLK2 inhibits the progression of gastric cancer by reprogramming amino acid metabolism through the mTOR/ASNS axis
Cancer Gene Therapy (2023)
-
Alterations in cellular metabolisms after Imatinib therapy: a review
Medical Oncology (2022)
-
High-resolution crystal structure of human asparagine synthetase enables analysis of inhibitor binding and selectivity
Communications Biology (2019)