Abstract
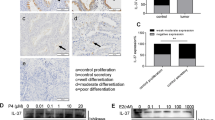
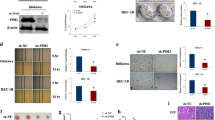
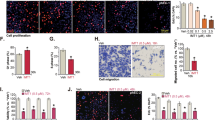
Researches regarding mitogen-inducible gene 6 (Mig-6) have confirmed its role as a tumor suppressor and progesterone resistance factor in endometrium. In this study, after confirming the downregulation of Mig-6 protein in endometrial carcinoma (EC) tissues, the expression of Mig-6 was upregulated in Ishikawa cells by pCMV6-Mig-6 plasmid. We observed the increased apoptosis, decreased proliferation and invasion potential of Ishikawa cells after upregulation of Mig-6. The proapoptosis ability of P4 significantly enhanced by 39.36%, the antiproliferation ability increased by 37.90% and the anti-invasion ability increased by 48.89%, suggesting the antiprogesterone resistance potential of Mig-6 in endometrium. In addition, the results suggested that Mig-6 may induce Ishikawa cell apoptosis through the mitochondrial pathway, inhibit cell proliferation via the extracellular signal-regulated kinase pathway and the anti-invasion potential may associate with matrix metalloproteinase (MMP)-2 and MMP-9 downexpression. Therefore, upregulation of Mig-6 may add a new strategy to suppress endometrial tumorigenesis and attenuate the progesterone resistance during P4 treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhang YW, Vande Woude GF . Mig-6, signal transduction, stress response and cancer. Cell Cycle 2007; 6: 507–513.
Hackel PO, Gishizky M, Ullrich A . Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem 2001; 382: 1649–1662.
Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B et al. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 2007; 26: 269–276.
Poetsch M, Woenckhaus C, Dittberner T, Pambor M, Lorenz G, Herrmann FH . Significance of the small subtelomeric area of chromosome 1 (1p36.3) in the progression of malignant melanoma: FISH deletion screening with YAC DNA probes. Virchows Arch 1999; 435: 105–111.
Nomoto S, Haruki N, Tatematsu Y, Konishi H, Mitsudomi T, Takahashi T et al. Frequent allelic imbalance suggests involvement of a tumor suppressor gene at 1p36 in the pathogenesis of human lung cancers. Genes Chromosomes Cancer 2000; 28: 342–346.
Anastasi S, Sala G, Huiping C, Caprini E, Russo G, Iacovelli S et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene 2005; 24: 4540–4548.
Ruan DT, Warren RS, Moalem J, Chung KW, Griffin AC, Shen W et al. Mitogen-inducible gene-6 expression correlates with survival and is an independent predictor of recurrence in BRAF(V600E) positive papillary thyroid cancers. Surgery 2008; 144: 908–914.
Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene 2010; 29: 3770–3780.
Li Z, Qu L, Zhong H, Xu K, Qiu X, Wang E . Low expression of Mig-6 is associated with poor survival outcome in NSCLC and inhibits cell apoptosis via ERK-mediated upregulation of Bcl-2. Oncol Rep 2014; 31: 1707–1714.
Xu J, Keeton AB, Wu L, Franklin JL, Cao X, Messina JL . Gene 33 inhibits apoptosis of breast cancer cells and increases poly(ADP-ribose) polymerase expression. Breast Cancer Res Treat 2005; 91: 207–215.
Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148: 3814–3826.
Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci USA 2009; 106: 8677–8682.
Zaman S, Wang R, Gandhi V . Targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma 2014; 55: 1980–1992.
Stacey DW . Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 2003; 15: 158–163.
Emmert-Buck MR, Roth MJ, Zhuang Z, Campo E, Rozhin J, Sloane BF et al. Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol 1994; 145: 1285–1290.
Fridman R, Toth M, Pena D, Mobashery S . Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res 1995; 55: 2548–2555.
McCawley LJ, Matrisian LM . Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 2000; 6: 149–156.
Ziel HK, Finkle WD . Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 1975; 293: 1167–1170.
Liang J, Shang Y . Estrogen and cancer. Annu Rev Physiol 2013; 75: 225–240.
Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med 2006; 12: 568–573.
Chen YC, Colvin ES, Maier BF, Mirmira RG, Fueger PT . Mitogen-inducible gene 6 triggers apoptosis and exacerbates ER stress-induced beta-cell death. Mol Endocrinol 2013; 27: 162–171.
Kim TH, Lee DK, Cho SN, Orvis GD, Behringer RR, Lydon JP et al. Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer Res 2013; 73: 5090–5099.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME . Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995; 270: 1326–1331.
Zhang B, Gu Y . Low expression of ERK signaling pathway affecting proliferation, cell cycle arrest and apoptosis of human gastric HGC-27 cells line. Mol Biol Rep 2014; 41: 3659–3669.
Li Z, Dong Q, Wang Y, Qu L, Qiu X, Wang E . Downregulation of Mig-6 in nonsmall-cell lung cancer is associated with EGFR signaling. Mol Carcinog 2012; 51: 522–534.
Lin CI, Barletta JA, Nehs MA, Morris ZS, Donner DB, Whang EE et al. Thyroid-specific knockout of the tumor suppressor mitogen-inducible gene 6 activates epidermal growth factor receptor signaling pathways and suppresses nuclear factor-kappaB activity. Surgery 2011; 150: 1295–1302.
Gotoh N . Feedback inhibitors of the epidermal growth factor receptor signaling pathways. Int J Biochem Cell Biol 2009; 41: 511–515.
Fiorini M, Ballaro C, Sala G, Falcone G, Alema S, Segatto O . Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene 2002; 21: 6530–6539.
Gupta GP, Massague J . Cancer metastasis: building a framework. Cell 2006; 127: 679–695.
Chambers AF, Matrisian LM . Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997; 89: 1260–1270.
Zucker S, Lysik RM, Zarrabi MH, Moll U . M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res 1993; 53: 140–146.
Crissman JD, Azoury RS, Barnes AE, Schellhas HF . Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol 1981; 57: 699–704.
Gallup DG, Stock RJ . Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol 1984; 64: 417–420.
Rackow BW, Arici A . Endometrial cancer and fertility. Curr Opin Obstet Gynecol 2006; 18: 245–252.
Lee WL, Lee FK, Su WH, Tsui KH, Kuo CD, Hsieh SL et al. Hormone therapy for younger patients with endometrial cancer. Taiwan J Obstet Gynecol 2012; 51: 495–505.
Park JY, Nam JH . Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist 2015; 20: 270–278.
Kim JJ, Chapman-Davis E . Role of progesterone in endometrial cancer. Semin Reprod Med 2010; 28: 81–90.
Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab 2011; 96: 1737–1746.
Al-Sabbagh M, Lam EW, Brosens JJ . Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol 2012; 358: 208–215.
Li X, Feng Y, Lin JF, Billig H, Shao R . Endometrial progesterone resistance and PCOS. J Biomed Sci 2014; 21: 2.
Aghajanova L, Velarde MC, Giudice LC . Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med 2010; 28: 51–58.
Miyamoto T, Watanabe J, Hata H, Jobo T, Kawaguchi M, Hattori M et al. Significance of progesterone receptor-A and -B expressions in endometrial adenocarcinoma. J Steroid Biochem Mol Biol 2004; 92: 111–118.
Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 2005; 146: 3490–3505.
Acknowledgements
This work was supported by Grant 81070471 from the National Natural Science Foundation of China and Grant RC201161 from Developing Health Engineering Fund Project of Jiangsu Province of China. We thank the State Key Laboratory of Reproduction Medicine at The First Affiliated Hospital of Nanjing Medical University for excellent technical assistance. This study was done in the Laboratory of Reproductive Medicine, The First Affiliated Hospital of Nanjing Medical University (also named Jiangsu Province Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, W., Zhu, S., Zhou, Y. et al. Upregulation of mitogen-inducible gene 6 triggers antitumor effect and attenuates progesterone resistance in endometrial carcinoma cells. Cancer Gene Ther 22, 536–541 (2015). https://doi.org/10.1038/cgt.2015.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2015.52
This article is cited by
-
ERRFI1 induces apoptosis of hepatocellular carcinoma cells in response to tryptophan deficiency
Cell Death Discovery (2021)
-
Fatostatin reverses progesterone resistance by inhibiting the SREBP1-NF-κB pathway in endometrial carcinoma
Cell Death & Disease (2021)
-
Panobinostat Enhances Growth Suppressive Effects of Progestin on Endometrial Carcinoma by Increasing Progesterone Receptor and Mitogen-Inducible Gene-6
Hormones and Cancer (2017)