Abstract
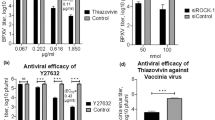
RhoA and its downstream effector Rho-associated coiled-coil-forming kinase (ROCK) are known regulators of the formation of actin cytoskeleton in cells. Actin cytoskeleton is involved in paramyxovirus infection; we, therefore, examined the effect of ROCK inhibition on measles virus (MV) cytopathic effect and replication. Treatment with the ROCK inhibitor, Y27632, significantly increased syncytia size in tumor cell lines following MV infection, associated with cytoskeleton disruption as demonstrated by actin staining. Treatment of prostate cancer, breast cancer and glioblastoma tumor cell lines with Y27632 following MV infection resulted in increased cytopathic effect, as assessed by trypan blue exclusion assays. In addition, there was a significant increase in viral proliferation by at least one log or more as tested in one-step viral growth curves. Increased viral replication was also observed in athymic nude mice bearing MDA-MB-231 xenografts following combination treatment with MV and Y27632. In summary, inhibition of the ROCK kinase by Y27632 enhanced the oncolytic effect of MV and viral proliferation; this approach merits further translational investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yanagi Y . The cellular receptor for measles virus--elusive no more. Rev Med Virol 2001; 11: 149–156.
Frenzke M, Sawatsky B, Wong XX, Delpeut S, Mateo M, Cattaneo R et al. Nectin-4-dependent measles virus spread to the cynomolgus monkey tracheal epithelium: role of infected immune cells infiltrating the lamina propria. J Virol 2013; 87: 2526–2534.
Muhlebach MD, Mateo M, Sinn PL, Prüfer S, Uhlig KM, Leonard VH et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011; 480: 530–533.
Iorio RM, Melanson VR, Mahon PJ . Glycoprotein interactions in paramyxovirus fusion. Future Virol 2009; 4: 335–351.
Navaratnarajah CK, Oezguen N, Rupp L, Kay L, Leonard VH, Braun W et al. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat Struct Mol Biol 2011; 18: 128–134.
Wild TF, Malvoisin E, Buckland R . Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol 1991; 72 (Pt 2): 439–442.
Smith EC, Popa A, Chang A, Masante C, Dutch RE . Viral entry mechanisms: the increasing diversity of paramyxovirus entry. FEBS J 2009; 276: 7217–7227.
Kallewaard NL, Bowen AL, Crowe JE Jr. . Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 2005; 331: 73–81.
Bedows E, Rao KM, Welsh MJ . Fate of microfilaments in vero cells infected with measles virus and herpes simplex virus type 1. Mol Cell Biol 1983; 3: 712–719.
Stallcup KC, Raine CS, Fields BN . Cytochalasin B inhibits the maturation of measles virus. Virology 1983; 124: 59–74.
Berghall H, Wallen C, Hyypia T, Vainionpaa R . Role of cytoskeleton components in measles virus replication. Arch Virol 2004; 149: 891–901.
Duprex WP, McQuaid S, Rima BK . Measles virus-induced disruption of the glial-fibrillary-acidic protein cytoskeleton in an astrocytoma cell line (U-251). J Virol 2000; 74: 3874–3880.
Wurth MA, Schowalter RM, Smith EC, Moncman CL, Dutch RE, McCann RO . The actin cytoskeleton inhibits pore expansion during PIV5 fusion protein-promoted cell-cell fusion. Virology 2010; 404: 117–126.
Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ . Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 1998; 17: 1415–1438.
Mukai M, Togawa A, Imamura F, Iwasaki T, Ayaki M, Mammoto T et al. Sustained tyrosine-phosphorylation of FAK through Rho-dependent adhesion to fibronectin is essential for cancer cell migration. Anticancer Res 2002; 22: 3175–3184.
Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S . An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med 1999; 5: 221–225.
Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol 2006; 26: 6844–6858.
Yoshioka K, Matsumura F, Akedo H, Itoh K . Small GTP-binding protein Rho stimulates the actomyosin system, leading to invasion of tumor cells. J Biol Chem 1998; 273: 5146–5154.
Imamura F, Mukai M, Ayaki M, Takemura K, Horai T, Shinkai K et al. Involvement of small GTPases Rho and Rac in the invasion of rat ascites hepatoma cells. Clin Exp Metastasis 1999; 17: 141–148.
Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E . ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 2006; 16: 1515–1523.
Chrzanowska-Wodnicka M, Burridge K . Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 1996; 133: 1403–1415.
Worthylake RA, Burridge K . RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem 2003; 278: 13578–13584.
Ashida N, Arai H, Yamasaki M, Kita T . Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem 2001; 276: 16555–16560.
Qiu RG, Chen J, McCormick F, Symons M . A role for Rho in Ras transformation. Proc Natl Acad Sci USA 1995; 92: 11781–11785.
Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer 1998; 77: 147–152.
Clark EA, Golub TR, Lander ES, Hynes RO . Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406: 532–535.
Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E . Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther 2009; 17: 1395–1403.
Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C, Rima BK . In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol 2000; 74: 7972–7979.
Msaouel P, Iankov ID, Allen C, Aderca I, Federspiel MJ, Tindall DJ et al. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol Ther 2009; 17: 2041–2048.
Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK . Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol 1999; 73: 9568–9575.
McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM et al. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat 2006; 99: 177–184.
Anderson BD, Nakamura T, Russell SJ, Peng KW . High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer research 2004; 64: 4919–4926.
Allen C, Opyrchal M, Aderca I, Schroeder MA, Sarkaria JN, Domingo E et al. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther 2012; 20: 444–449.
Opyrchal M, Allen C, Iankov I, Aderca I, Schroeder M, Sarkaria J et al. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS). Hum Gene Ther 2012; 23: 419–427.
Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther 2008; 16: 1556–1564.
Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther 2008; 8: 213–220.
Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res 2007; 13: 7155–7165.
Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther 2007; 15: 677–686.
Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res 2006; 66: 11840–11850.
Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat 2010; 122: 745–754.
Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004; 103: 1641–1646.
Studebaker AW, Kreofsky CR, Pierson CR, Russell SJ, Galanis E, Raffel C . Treatment of medulloblastoma with a modified measles virus. Neuro Oncol 2010; 12: 1034–1042.
Studebaker AW, Hutzen B, Pierson CR, Russell SJ, Galanis E, Raffel C . Oncolytic measles virus prolongs survival in a murine model of cerebral spinal fluid-disseminated medulloblastoma. Neuro Oncol 2012; 14: 459–470.
Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R et al. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009; 69: 82–91.
Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006; 44: 1465–1477.
Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ . Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res 2002; 62: 4656–4662.
Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 2010; 70: 875–882.
Ulloa L, Serra R, Asenjo A, Villanueva N . Interactions between cellular actin and human respiratory syncytial virus (HRSV). Virus Res 1998; 53: 13–25.
Giuffre RM, Tovell DR, Kay CM, Tyrrell DL . Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol 1982; 42: 963–968.
Burke E, Mahoney NM, Almo SC, Barik S . Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J Virol 2000; 74: 669–675.
Wang JL, Zhang JL, Chen W, Xu XF, Gao N, Fan DY et al. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl Trop Dis 2010; 4: pii: e809.
Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 2000; 57: 976–983.
Nishimura Y, Itoh K, Yoshioka K, Tokuda K, Himeno M . Overexpression of ROCK in human breast cancer cells: evidence that ROCK activity mediates intracellular membrane traffic of lysosomes. Pathol Oncol Res 2003; 9: 83–95.
de Toledo M, Anguille C, Roger L, Roux P, Gadea G . Cooperative anti-invasive effect of Cdc42/Rac1 activation and ROCK inhibition in SW620 colorectal cancer cells with elevated blebbing activity. PLoS One 2012; 7: e48344.
Lawler K, Foran E, O'Sullivan G, Long A, Kenny D . Mobility and invasiveness of metastatic esophageal cancer are potentiated by shear stress in a ROCK- and Ras-dependent manner. Am J Physiol Cell Physiol 2006; 291: C668–C677.
Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A et al. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res 2005; 65: 8792–8800.
Sahai E, Ishizaki T, Narumiya S, Treisman R . Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol 1999; 9: 136–145.
Xu XT, Song QB, Yao Y, Ruan P, Tao ZZ . Inhibition of RhoA/ROCK signaling pathway promotes the apoptosis of gastric cancer cells. Hepatogastroenterology 2012; 59: 2523–2526.
Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res 2011; 39: D945–D950.
Fritz G, Just I, Kaina B . Rho GTPases are over-expressed in human tumors. Int J Cancer 1999; 81: 682–687.
Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP et al. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res 2012; 72: 5025–5034.
Nakajima M, Hayashi K, Egi Y, Katayama K, Amano Y, Uehata M et al. Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16 melanoma. Cancer Chemother Pharmacol 2003; 52: 319–324.
Takamura M, Sakamoto M, Genda T, Ichida T, Asakura H, Hirohashi S . Inhibition of intrahepatic metastasis of human hepatocellular carcinoma by Rho-associated protein kinase inhibitor Y-27632. Hepatology 2001; 33: 577–581.
Acknowledgements
This work was supported in part by NIH grants P50CA136393, P50108961, R01 CA136547 and R01 CA154348 and an Atwater grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Opyrchal, M., Allen, C., Msaouel, P. et al. Inhibition of Rho-associated coiled-coil-forming kinase increases efficacy of measles virotherapy. Cancer Gene Ther 20, 630–637 (2013). https://doi.org/10.1038/cgt.2013.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.58