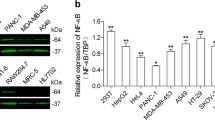
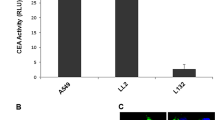
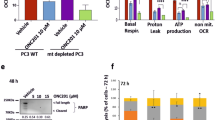
Abstract
We previously developed an artificially constructed promoter that was activated in response to X-ray irradiation in LNCap, a prostate cancer cell line. Anticancer drugs were examined to see whether some of them could stimulate the activity of the promoter. It was found that doxorubicin (Dox) treatment to LNCap transfected with a gene cassette of the luciferase gene under control of the promoter-enhanced luciferase activity in a dose-dependent manner, indicating that the promoter could be controlled by Dox. When the luciferase gene was replaced with the fcy::fur gene whose product facilitates conversion of 5-fluorocytosine into 5-fluorouracil that is highly toxic, Dox stimulated the expression of the gene product, resulting in facilitation of cell killing effect in the presence of 5-fluorocytosine. These results suggest that therapeutic gene expression controlled with an anticancer drug may lead to a more effective cancer therapy with less hazardous side effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Oliver SE, May MT, Gunnell D . International trends in prostate-cancer mortality in the ‘PSA era’. Int J Cancer 2001; 92: 893–898.
Immediate versus deferred treatment for advanced prostate cancer: initial result of the Medical Research Council Trial. Br J Urol 1997; 79: 235–246.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Petrylak DP, Tangen CM, Hussain MHA, Lara Jr PN, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Kikuno N, Urakami S, Nakamura S, Hiraoka T, Hyuga T, Arichi N et al. Phase-II study of docetaxel, estramustine phosphate, and carboplatin in patients with hormone-refractory prostate cancer. Eur Urol 2007; 51: 1252–1258.
Morii A, Ogawa R, Watanabe A, Kakutani S, Zhao QL, Kume K et al. Regulation of gene expression in prostate cancer cells with an artificially constructed promoter responsive to radiation. Gene Ther 2012; 19: 219–227.
Scott SD, Greco O . Radiation and hypoxia inducible gene therapy systems. Cancer Metastasis Rev 2004; 23: 269–276.
Cao XM, Guy GR, Sukhatme VP, Tan YH . Regulation of the Egr-1 gene by tumor necrosis factor and interferons in primary human fibroblasts. J Biol Chem 1992; 267: 1345–1349.
Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J . Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, egr-1, andjunB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 11998; 273: 31327–31336.
Datta R, Rubin E, Sukhatme V, Qureshi S, Hallahan D, Weichselbaum RR et al. Ionizing radiation activates transcription of the EGR1 gene via CArG elements. Proc Natl Acad Sci USA 1992; 89: 10149–10153.
Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R, Kufe DW . Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA 1993; 90: 2419–2422.
Park JO, Lopez CA, Gupta VK, Brown CK, Mauceri HJ, Darga TE et al. Transcriptional control of viral gene therapy by cisplatin. J Clin Invest 2002; 110: 403–410.
Greco O, Powell TM, Marples B, Joiner MC, Scott SD . Gene therapy vectors containing CArG elements from the Egr1 gene are activated by neutron irradiation, cisplatin and doxorubicin. Cancer Gene Ther 2005; 12: 655–662.
Quiñones A, Dobberstein KU, Rainov NG . The egr-1 gene is induced by DNA-damaging agents and non-genotoxic drugs in both normal and neoplastic human cells. Life Sci 2003; 72: 2975–2992.
Steiner MS, Gungrich JR . Gene therapy for prostate cancer: where are we now? J Urol 2000; 164: 1121–1136.
Sambrook J, Russell DW . Molecular Cloning: A Laboratory Manual, 3rd edn.Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY. 2001.
Gewirtz DA . A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727–741.
Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF . Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 1997; 280: 638–649.
Hanly L, Chen N, Rieder M, Koren G . Ifosfamide nephrotoxicity in children: a mechanistic base for pharmacological prevention. Expert Opin Drug Saf 2009; 8: 155–168.
Rabi T, Bishayee A . d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinogen 2009; 8: 9.
Doroshow JH, Akman S, Chu FF, Esworthy S . Role of the glutathione-glutathione peroxidase cycle in the cytotoxicity of the anticancer quinones. Pharmacol Ther 1990; 47: 359–370.
Wang YF, Chen CY, Chung SF, Chiou YH, Lo HR . Involvement of oxidative stress and caspase activation in paclitaxel-induced apoptosis of primary effusion lymphoma cells. Cancer Chemother Pharmacol 2004; 54: 322–330.
Petrioli R, Fiaschi AI, Francini E, Pascucci A, Francini G . The role of doxorubicin and epirubicin in the treatment of patients with metastatic hormone-refractory prostate cancer. Cancer Treat Rev 2008; 34: 710–718.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (C) (21500403) and for Young Scientist (B) (20377253 and 23791746) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ogawa, R., Morii, A., Watanabe, A. et al. An artificially constructed radiation-responsive promoter is activated by doxorubicin. Cancer Gene Ther 19, 345–351 (2012). https://doi.org/10.1038/cgt.2012.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.7