Abstract
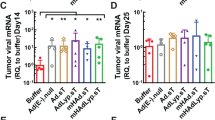
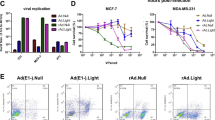
We have examined the effect of adenoviruses expressing soluble transforming growth factor receptorII-Fc (sTGFβRIIFc) in a 4T1 mouse mammary tumor bone metastasis model using syngeneic BALB/c mice. Infection of 4T1 cells with a non-replicating adenovirus, Ad(E1−).sTβRFc, or with two oncolytic adenoviruses, Ad.sTβRFc and TAd.sTβRFc, expressing sTGFβRIIFc (the human TERT promoter drives viral replication in TAd.sTβRFc) produced sTGFβRIIFc protein. Oncolytic adenoviruses produced viral replication and induced cytotoxicity in 4T1 cells. 4T1 cells were resistant to the cytotoxic effects of TGFβ-1 (up to 10 ng ml−1). However, TGFβ-1 induced the phosphorylation of SMAD2 and SMAD3, which were inhibited by co-incubation with sTGFβRIIFc protein. TGFβ-1 also induced interleukin-11, a well-known osteolytic factor. Intracardiac injection of 4T1-luc2 cells produced bone metastases by day 4. Intravenous injection of Ad.sTβRFc (on days 5 and 7) followed by bioluminescence imaging (BLI) of mice on days 7, 11 and 14 in tumor-bearing mice indicated inhibition of bone metastasis progression (P<0.05). X-ray radiography of mice on day 14 showed a significant reduction of the lesion size by Ad.sTβRFc (P<0.01) and TAd.sTβRFc (P<0.05). Replication-deficient virus Ad(E1−).sTβRFc expressing sTGFβRIIFc showed some inhibition of bone metastasis, whereas Ad(E1−).Null was not effective in inhibiting bone metastases. Thus, systemic administration of Ad.sTβRFc and TAd.sTβRFc can inhibit bone metastasis in the 4T1 mouse mammary tumor model, and can be developed as potential anti-tumor agents for breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cancer Facts and Figures 2011. American Cancer Society. http://www.cancer.org/Research/cancer-facts-figures-2011.
Coleman RE . Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27: 165–176.
Candelaria-Quintana D, Dayao ZR, Royce ME . The role of antiresorptive therapies in improving patient care in early and metastatic breast cancer. Breast Cancer Res Treat 2012; 132: 355–363.
Crompton AM, Kirn DH . From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets 2007; 7: 133–139.
Toth K, Dhar D, Wold WS . Oncolytic (replication-competent) adenoviruses as anticancer agents. Expert Opin Biol Ther 2010; 10: 353–368.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996; 274: 373–376.
Reid T, Warren R, Kirn D . Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002; 9: 979–986.
Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Therapy 2001; 8: 746–759.
Nemunaitis J, Tong AW, Nemunaitis M, Senzer N, Phadke AP, Bedell C et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther 2010; 18: 429–434.
Seth P, Wang ZG, Pister A, Zafar MB, Kim S, Guise T et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factor-beta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther 2006; 17: 1152–1160.
Hu Z, Zhang Z, Guise T, Seth P . Systemic Delivery of an Oncolytic Adenovirus Expressing Soluble Transforming Growth Factor-beta Receptor II-Fc Fusion Protein Can Inhibit Breast Cancer Bone Metastasis in a Mouse Model. Hum Gene Ther 2010; 21: 1623–1629.
Hu Z, Gerseny H, Zhang Z, Chen YJ, Berg A, Stock S et al. Oncolytic Adenovirus Expressing Soluble TGFbeta Receptor II-Fc-mediated Inhibition of Established Bone Metastases: A Safe and Effective Systemic Therapeutic Approach for Breast Cancer. Mol Ther 2011; 9: 1609–1618.
Teicher BA . Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev 2001; 20: 133–143.
Barcellos-Hoff MH, Akhurst RJ . Transforming growth factor-beta in breast cancer: too much, too late. Breast Cancer Res 2009; 11: 202.
Padua D, Massague J . Roles of TGFbeta in metastasis. Cell Res 2009; 19: 89–102.
Buijs JT, Stayrook KR, Guise TA . TGF-beta in the Bone Microenvironment: Role in Breast Cancer Metastases. Cancer Microenviron 2011; 4: 261–281.
Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006; 12 (20 Part 2): 6213s–6216s.
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A 2005; 102: 13909–13914.
Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P . Biology of breast cancer bone metastasis. Cancer Biol Ther 2007; 7: 3–9.
Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 2002; 109: 1551–1559.
Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest 2002; 109: 1607–1615.
Iyer I, Wang Z-G, Akhtari M, Zhao W, Seth P . Targeting TGF beta signaling for cancer therapy. Cancer Biol Ther 2005; 4: 261–266.
Nagaraj NS, Datta PK . Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs 2010; 19: 77–91.
Juarez P, Guise TA . TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone 2011; 48: 23–29.
Hu Z, Robbins JS, Pister A, Zafar MB, Zhang ZW, Gupta J et al. A modified hTERT promoter-directed oncolytic adenovirus replication with concurrent inhibition of TGFbeta signaling for breast cancer therapy. Cancer Gene Ther 2010; 17: 235–243.
Katayose D, Gudas J, Nguyen H, Srivastava S, Cowan KH, Seth P . Cytotoxic effects of adenovirus-mediated wild-type p53 protein expression in normal and tumor mammary epithelial cells. Clin Cancer Res 1995; 1: 889–897.
Zhang Z, Krimmel J, Hu Z, Seth P . Systemic Delivery of a Novel Liver-Detargeted Oncolytic Adenovirus Causes Reduced Liver Toxicity but Maintains the Antitumor Response in a Breast Cancer Bone Metastasis Model. Hum Gene Ther 2011; 22: 1137–1142.
Gupta J, Robbins J, Jilling T, Seth P . TGFbeta-dependent induction of Interleukin-11 and Interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther 2011; 11: 311–116.
Nguyen DX, Massague J . Genetic determinants of cancer metastasis. Nat Rev Genet 2007; 8: 341–352.
Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y . Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med 2009; 15: 960–966.
Guo W, Zhu H, Zhang L, Davis J, Teraishi F, Roth JA et al. Combination effect of oncolytic adenovirotherapy and TRAIL gene therapy in syngeneic murine breast cancer models. Cancer Gene Ther 2006; 13: 82–90.
Doherty GJ, McMahon HT . Mechanisms of endocytosis. Annu Rev Biochem 2009; 78: 857–902.
Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008; 132: 397–409.
Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS . Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther 2008; 16: 1665–1673.
Bessis N, GarciaCozar FJ, Boissier MC . Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Therapy 2004; 11 (Suppl 1): S10–S17.
Ahi YS, Bangari DS, Mittal SK . Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 2011; 11: 307–320.
Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 2008; 68: 3915–3923.
Acknowledgements
The research described here was funded by a grant from the National Cancer Institutes Grant #R01CA127380 (PS). We are thankful to Janardan Khandekar, Theodore Mazzone, Bruce Brockstein and an anonymous source for their generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Z., Hu, Z., Gupta, J. et al. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther 19, 630–636 (2012). https://doi.org/10.1038/cgt.2012.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.41
Keywords
This article is cited by
-
The safety and efficacy of systemic delivery of a new liver-de-targeted TGFβ signaling inhibiting adenovirus in an immunocompetent triple negative mouse mammary tumor model
Cancer Gene Therapy (2024)
-
The limiting factors of oncolytic virus immunotherapy and the approaches to overcome them
Applied Microbiology and Biotechnology (2020)
-
A novel immunocompetent murine model for replicating oncolytic adenoviral therapy
Cancer Gene Therapy (2015)
-
The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer
Gene Therapy (2015)
-
Combinatorial treatment with oncolytic adenovirus and helper-dependent adenovirus augments adenoviral cancer gene therapy
Molecular Therapy - Oncolytics (2014)