Abstract
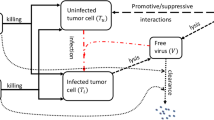
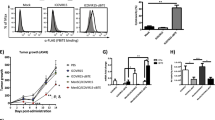
Treatment of metastatic tumors with engineered adenoviruses that replicate selectively in tumor cells is a new therapeutic approach in cancer. Systemic administration of these oncolytic adenoviruses lack metastatic targeting ability. The tumor stroma engrafting property of intravenously injected mesenchymal stem cells (MSCs) may allow the use of MSCs as cellular vehicles for targeted delivery. In this work, we study the safety and the efficacy of infusing autologous MSCs infected with ICOVIR-5, a new oncolytic adenovirus, for treating metastatic neuroblastoma. Four children with metastatic neuroblastoma refractory to front-line therapies received several doses of autologous MSCs carrying ICOVIR-5, under an approved preliminary study. The tolerance to the treatment was excellent. A complete clinical response was documented in one case, and the child is in complete remission 3 years after this therapy. We postulate that MSCs can deliver oncolytic adenoviruses to metastatic tumors with very low systemic toxicity and with beneficial antitumor effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Maris JM, Hogarty MD, Bagatell R, Cohn SL . Neuroblastoma. Lancet 2007; 369: 2106–2120.
Alemany R . Cancer selective adenoviruses. Mol Aspects Med 2007; 28: 42–58.
Power AT, Bell JC . Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther 2007; 15: 660–665.
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M . Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 2002; 62: 3603–3608.
Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat 2007; 105: 157–167.
Pereboeva L, Komarova S, Mikheeva G, Krasnykh V, Curiel DT . Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells 2003; 21: 389–404.
Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L . Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Chen JMol Cancer Ther 2006; 5: 755–766.
Nakamizo A, Marini F, Amano T, Studeny M, Gumin J, Chen J et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 2005; 65: 3307–3318.
Thorne SH, Negrin RS, Contag CH . Synergistic antitumor effects of immune cellviral biotherapy. Science 2006; 311: 1780–1784.
Alemany R, Balagué C, Curiel DT . Replicative adenoviruses for cancer therapy. Nat Biotechnol 2000; 18: 723–727.
Cascallo M, Alonso MM, Rojas JJ, Perez-Gimenez A, Fueyo J, Alemany R . Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol Ther 2007; 15: 1607–1615.
Alonso MM, Cascallo M, Gomez-Manzano C, Jiang H, Bekele BN, Perez-Gimenez A et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res 2007; 67: 8255–8263.
Dmitriev I, Krasnykh V, Millar CR, Wang M, Kashentseva E, Mikheeva G et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 1998; 72: 9706–9713.
Alemany R, Curiel DT . CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Therapy 2001; 8: 1347–1353.
García-Castro J, Balas A, Ramírez M, Pérez-Martínez A, Madero L, González-Vicent M et al. Mesenchymal stem cells are of recipient origin in pediatric transplantations using umbilical cord blood, peripheral blood, or bone marrow. J Pediatr Hematol Oncol 2007; 29: 388–392.
Majem M, Cascallo M, Bayo-Puxan N, Mesia R, Germa JR, Alemany R . Control of E1A under an E2F-1 promoter insulated with the myotonic dystrophy locus insulator reduces the toxicity of oncolytic adenovirus Ad-Delta24RGD. Cancer Gene Ther 2006; 13: 696–705.
Avellón A, Pérez P, Aguilar JC, Lejarazu R, Echevarría JE . Rapid and sensitive diagnosis of human adenovirus infections by a generic polymerase chain reaction. J Virol Methods 2001; 92: 113–120.
Wadell G, Allard A, Hierholzer JC . Adenoviruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds). Manual of Clinical Microbiology. 7th edn. American Society for Microbiology: Washington DC, 1999. pp 970–982.
Navarro S, González-Devesa M, Ferrández-Izquierdo A, Triche TJ, Llombart-Bosch A . Scanning electron microscopic evidence for neural differentiation in Ewing′s sarcoma cell lines. Virchows Arch A Pathol Anat Histopathol 1990; 416: 383–391.
Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a Pilot Study. Iran J Immunol 2007; 4: 50–57.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767.
Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv 2005; 65: 321–329.
Bang OY, Lee JS, Lee PH, Lee G . Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 57: 874–882.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W . Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215–222.
Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001; 97: 1227–1231.
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–563.
Post DE, Khuri FR, Simons JW, Van Meir EG . Replicative oncolytic adenoviruses in multimodal cancer regimens. Hum Gene Ther 2003; 14: 933–946.
Matzinger P . The danger model: a renewed sense of self. Science 2002; 296: 301–305.
Parato KA, Senger D, Forsyth PA, Bell JC . Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005; 5: 965–976.
Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med 2008; 14: 37–44.
Fish JD, Grupp SA . Stem cell transplantation for neuroblastoma. Bone Marrow Transplant 2008; 41: 159–165.
Aghi M, Martuza RL . Oncolytic viral therapies: the clinical experience. Oncogene 2005; 24: 7802–7816.
Pinkerton CR, Blanc Vincent MP, Bergeron C, Fervers B, Philip T . Induction chemotherapy in metastatic neuroblastoma–does dose influence response? A critical review of published data standards, options and recommendations (SOR) project of the National Federation of French Cancer Centres (FNCLCC). Eur J Cancer 2000; 36: 1808–1815.
Acknowledgements
We thank Dr Samuel Navarro for help with electronic microscopy, Dr José Díaz for help with MIBG interpretation and Jaime Valentín for technical assistance. Financial support: JG-C supported by Grant FIS PI05/2217 (Instituto de Salud Carlos III) and TCRM 0027/2006 (Junta de Andalucía). MC supported by grant from Mutua Madrileña Medical Research Foundation. RA supported by EU 6th FP research contract 18700 (Theradpox, RA) and project Grant BIO2005-08682-C03-02/01 from the Spanish Ministry of Education and Science. RA belongs to the Network of Cooperative Research on Cancer (C03-10), Instituto de Salud Carlos III of the Ministerio de Sanidad y Consumo, Government of Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Rights and permissions
About this article
Cite this article
García-Castro, J., Alemany, R., Cascalló, M. et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther 17, 476–483 (2010). https://doi.org/10.1038/cgt.2010.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.4
Keywords
This article is cited by
-
AKT and JUN are differentially activated in mesenchymal stem cells after infection with human and canine oncolytic adenoviruses
Cancer Gene Therapy (2021)
-
Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy
Scientific Reports (2020)
-
Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials
Clinical and Translational Medicine (2018)
-
Antitumor virotherapy using syngeneic or allogeneic mesenchymal stem cell carriers induces systemic immune response and intratumoral leukocyte infiltration in mice
Cancer Immunology, Immunotherapy (2018)
-
Gene therapy as a potential tool for treating neuroblastoma—a focused review
Cancer Gene Therapy (2016)