Abstract
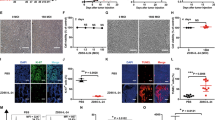
It has been shown that interleukin 18 (IL-18) exerts antitumor activity. In this study, we investigated whether oncolytic adenovirus-mediated gene transfer of IL-18 could induce strong antitumor activity. A tumor-selective replicating adenovirus expressing IL-18 (ZD55-IL-18) was constructed by insertion of an IL-18 expression cassette into the ZD55 vector, which is based on deletion of the adenoviral E1B 55-kDa gene. It has been shown that ZD55-IL-18 exerted a strong cytopathic effect and significant apoptosis in tumor cells. ZD55-IL-18 significantly decreased vascular endothelial growth factor and CD34 expression in the melanoma cells. Treatment of established tumors with ZD55-IL-18 showed much stronger antitumor activity than that induced by ZD55-EGFP (enhanced green fluorescent protein) or Ad-IL-18. These data indicated that oncolytic adenovirus expressing IL-18 could exert potential antitumor activity through inhibition of angiogenesis and offer a novel approach to melanoma therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- EGFP:
-
enhanced green fluorescent protein
- IL-18:
-
interleukin 18
- MOI:
-
multiplicity of infection
- TUNEL:
-
TdT-mediated dUTP-biotin nick end-labeling assay
- VEGF:
-
vascular endothelial growth factor
References
Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995; 378: 88–91.
Smyth MJ, Taniguchi M, Street SE . The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol 2000; 165: 2665–2670.
Okamoto I, Kohno K, Tanimoto T, Ikegami H, Kurimoto M . Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J Immunol 1999; 162: 3202–3211.
Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H . Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell Immunol 1996; 173: 230–235.
Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M et al. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol 1996; 26: 1647–1651.
Okano F, Yamada K . Canine interleukin-18 induces apoptosis and enhances Fas ligand mRNA expression in a canine carcinoma cell line. Anticancer Res 2000; 20: 3411–3415.
Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest 1998; 101: 1441–1452.
Micallef MJ, Tanimoto T, Kohno K, Ikeda M . Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res 1997; 57: 4557–4563.
Osaki T, Péron JM, Cai Q, Okamura H, Robbins PD, Kurimoto M et al. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol 1998; 160: 1742–1749.
Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S et al. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas–Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol 1999; 163: 583–589.
Wigginton JM, Lee JK, Wiltrout TA, Alvord WG, Hixon JA, Subleski J et al. Synergistic engagement of an ineffective endogenous anti-tumor immune response and induction of IFN-gamma and Fas-ligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J Immunol 2002; 169: 4467–4474.
Akamatsu S, Arai N, Hanaya T, Arai S, Tanimoto T, Fujii M et al. Antitumor activity of interleukin-18 against the murine T-cell leukemia/lymphoma EL-4 in syngeneic mice. J Immunother 2002; 25: S28–S34.
Wang Q, Yu H, Ju DW, He L, Pan JP, Xia DJ et al. Intratumoral IL-18 gene transfer improves therapeutic efficacy of antibody-targeted superantigen in established murine melanoma. Gene Ther 2001; 8: 542–550.
Ju DW, Yang Y, Tao Q, Song WG, He L, Chen G et al. Interleukin-18 gene transfer increases antitumor effects of suicide gene therapy through efficient induction of antitumor immunity. Gene Ther 2000; 7: 1672–1679.
Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res 2006; 12: 4265–4273.
Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K et al. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res 1998; 4: 1183–1191.
Portielje JE, Kruit WH, Schuler M, Beck J, Lamers CH, Stoter G et al. Phase I study of subcutaneously administered recombinant human interleukin 12 in patients with advanced renal cell cancer. Clin Cancer Res 1999; 5: 3983–3989.
Carson WE, Dierksheide JE, Jabbour S, Anghelina M, Bouchard P, Ku G et al. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-gamma production and STAT-mediated signal transduction. Blood 2000; 96: 1465–1473.
Osaki T, Hashimoto W, Gambotto A, Okamura H, Robbins PD, Kurimoto M et al. Potent antitumor effects mediated by local expression of the mature form of the interferon-gamma inducing factor, interleukin-18 (IL-18). Gene Ther 1999; 6: 808–815.
Dobbelstein M . Replicating adenoviruses in cancer therapy. Curr Top Microbiol Immunol 2004; 273: 291–334.
Chu RL, Post DE, Khuri FR, Van Meir EG . Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res 2004; 10: 5299–5312.
Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH . ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 1997; 3: 639–645.
Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst 2003; 95: 652–660.
Kirn D . Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned. Gene Ther 2001; 8: 89–98.
Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther 2001; 8: 746–759.
Van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR . Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res 2002; 62: 6165–6171.
Wadler S, Yu B, Tan JY, Kaleya R, Rozenblit A, Makower D et al. Persistent replication of the modified chimeric adenovirus ONYX-015 in both tumor and stromal cells from a patient with gall bladder carcinoma implants. Clin Cancer Res 2003; 9: 33–43.
Osaki T, Péron JM, Cai Q, Okamura H, Robbins PD, Kurimoto M et al. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol 1998; 160: 1742–1749.
Redondo P, Sánchez-Carpintero I, Bauzá A, Idoate M, Solano T, Mihm Jr MC . Immunologic escape and angiogenesis in human malignant melanoma. J Am Acad Dermatol 2003; 49: 255–263.
Folkman J . What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6.
Rofstad EK, Halsør EF . Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res 2000; 60: 4932–4938.
Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Müller M et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res 2000; 60: 4819–4824.
Cao R, Farnebo J, Kurimoto M, Cao Y . Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J 1999; 13: 2195–2202.
Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE . Evidence of IL-18 as a novel angiogenic mediator. J Immunol 2001; 167: 1644–1653.
Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH et al. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett 2006; 103: 159–166.
Kim J, Kim C, Kim TS, Bang SI, Yang Y, Park H et al. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Commun 2006; 344: 1284–1289.
Zhang Q, Nie M, Sham J, Su C, Xue H, Chua D et al. Effective gene-viral therapy for telomerase-positive cancers by selective replicative-competent adenovirus combining with endostatin gene. Cancer Res 2004; 64: 5390–5397.
Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res 2001; 61: 5453–5460.
Micallef MJ, Yoshida K, Kawai S, Hanaya T, Kohno K, Arai S et al. In vivo antitumor effects of murine interferon-gamma-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother 1997; 43: 361–367.
Nguyen M, Branton PE, Roy S, Nicholson DW, Alnemri ES, Yeh WC et al. E1A-induced processing of procaspase-8 can occur independently of FADD and is inhibited by Bcl-2. J Biol Chem 1998; 273: 33099–33102.
Querido E, Teodoro JG, Branton PE . Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J Virol 1997; 71: 3526–3533.
Gu W, Putral L, Hengst K, Minto K, Saunders NA, Leggatt G et al. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther 2006; 13: 1023–1032.
Acknowledgements
This project is supported by grants from the National Natural Science Foundation of China (No. 30700999), the Science and Technology Department of Jiangsu province (No. BK2006036) and the Program for New Century Excellent Talents in University (NCET-08-0700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, JN., Pei, DS., Mao, LJ. et al. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther 17, 28–36 (2010). https://doi.org/10.1038/cgt.2009.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.38
Keywords
This article is cited by
-
Water/ethanol extract of Cucumis sativus L. fruit attenuates lipopolysaccharide-induced inflammatory response in endothelial cells
BMC Complementary and Alternative Medicine (2018)
-
Systemically administered liposome-encapsulated Ad-PEDF potentiates the anti-cancer effects in mouse lung metastasis melanoma
Journal of Translational Medicine (2013)
-
Recent developments in oncolytic adenovirus-based immunotherapeutic agents for use against metastatic cancers
Cancer Gene Therapy (2013)
-
Clinical value of serum interleukin-18 and nitric oxide activities in patients with prostate cancer
The Chinese-German Journal of Clinical Oncology (2011)