Abstract
Circulating tumour cells (CTCs), identified in numerous cancers including melanoma, are unquestionably considered valuable and useful as diagnostic and prognostic markers. They can be detected at all melanoma stages and may persist long after treatment. A crucial step in metastatic processes is the intravascular invasion of neoplastic cells as circulating melanoma cells (CMCs). Only a small percentage of these released cells are efficient and capable of colonizing with a strong metastatic potential. CMCs' ability to survive in circulation express a variety of genes with continuous changes of signal pathways and proteins to escape immune surveillance. This makes it difficult to detect them; therefore, specific isolation, enrichment and characterization of CMC population could be useful to monitor disease status and patient clinical outcome. Overall and disease-free survival have been correlated with the presence of CMCs. Specific melanoma antigens, in particular MCAM (MUC18/MelCAM/CD146), could be a potentially useful tool to isolate CMCs as well as be a prognostic, predictive biomarker. These are the areas reviewed in the article.
Similar content being viewed by others
Facts:
-
Circulating tumour cells (CTCs) are detectable at all stages of disease and persist long after treatment.
-
CTCs are detectable, even when patients are considered disease-free, using antibodies against specific cell surface markers.
-
Circulating melanoma cells (CMCs) are thought to be responsible for metastatic progression, making it especially lethal.
-
Certain CMCs could be useful to monitor the disease status.
-
Melanoma does not express the classical epithelial cell surface markers, such as EpCAM, which has formed the basis of most CTC isolation strategies.
-
Expression of melanoma cell-adhesion molecule MCAM (MUC18/MelCAM/CD146) is associated with an aggressive, invasive phenotype and its upregulation is strongly associated with disease progression.
Open Questions:
-
How can CMCs be isolated? Which genotype and phenotype do they express?
-
Have all CMC phenotypes a metastatic potential? Which phenotype should be considered dangerous?
-
As their detection in melanoma patients is useful to monitor the disease status, can CMCs have a prognostic value?
-
Can the CMCs' enumeration and characterization determine and predict the overall and disease-free survival?
-
Can MCAM (MUC18/MelCAM/CD146), as one of the most melanoma-specific cell-surface epitope, be regarded either as a useful tool to isolate CMCs or as a possible prognostic or predictive biomarker of melanoma progression?
Circulating Tumour Cells
In cancer, the metastatic spreading of cells from a primary tumour to distant sites is mostly responsible for patient morbidity and mortality.1 Millions of cells are shed from primary tumour every day (approximately 4×106 cells/g primary tumour)2,3 invading lymphatic and/or venous circulatory systems. Once they enter into the bloodstream, they become circulating tumour cell population (CTCs).
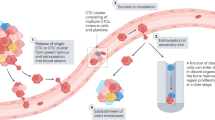
Fortunately, in cancer metastasis, only a small percentage of released cells are efficient and capable of colonizing as well as forming distant lesions, as survival can be limited by immune surveillance or haemodynamic forces.4,5 Experimental studies documented that only 0.01% of cancer cells injected into circulation form metastatic foci.5,6 It is not clear at which steps of the process the cells are lost, but the fact that metastasis can occur many years later after diagnosis and surgery indicates that most disseminated CTCs have been rapidly destroyed in the circulation or that they may remain apparently quiescent. Nevertheless, the presence of CTCs is a prerequisite step to establish distant metastases during the course of a given cancer and these cells are considered appropriately as the ‘leukaemic’ phase of solid tumour.7 CTCs acquire activating changes that lead to their extravasation into the surrounding tissue, degradation of basement membrane and extracellular matrix, capacity to migrate, adhere and propagate via the lymphatic and circulatory systems and establish new colonies at distant sites that will lead to metastatic disease.7 In one cubic centimetre of tissue, it has been established there are 109 cells that rapidly can reach 1010–1011 cells before becoming clinically detectable.8 The ability to measure CTCs represents a potential powerful method to monitor patients with known malignancies who have a minimal morbidity. Despite metastasis is a relatively inefficient process, researchers and clinicians believe that CTCs can be effectively used to screen early-stage cancers. Detection of these CTC micrometastases seems to be useful for patient stratification groups with low or high risk for metastatic disease9–16 and as an independent prognostic marker in a variety of metastatic cancer.17–23 CTCs number, prior to initiation, during and after therapy, has been shown to be indicative of the length of progression-free survival (PFS) and overall survival (OS);17,19,21,23–25 moreover, its monitoring during and after therapy showed a correlation with the clinical course of the disease and the response to therapy.17,22,26,27 Predictive value for survival, based on CTC enumeration has been shown to be superior to standard monitoring tests.18,28,29 Detection, genotyping, phenotyping and molecular characterization of CTCs and more recently of cell-free circulating nucleic acids (in particular, circulating tumour DNA, ctDNA) have introduced the ‘new concept’ of ‘liquid biopsy’. ctDNA fragments mainly originate from apoptotic or necrotic tumour cells, which discharge their DNA into the blood circulation in several malignancies. They have proven to be of prognostic value in several cancers and have been detected with a sensitivity ranging from 26 to 100% analysing different genetic markers.30 Although molecular targets were initially detected in nucleic acid samples extracted from tumour tissue, the detection of nucleic acids in circulating blood has allowed alternative sample source for the identification of genomic alterations. Biopsy of overt metastases is an invasive procedure limited to certain locations and not easily acceptable in the clinic; 'liquid biopsy’ can be conducted serially and might allow real-time monitoring of cancer therapies in individual patients. Both CTCs and ctDNA are interesting complementary technologies that can be used in parallel in future trials assessing new drugs or drug combinations and may provide clinically relevant information that could rapidly revolutionize oncology practice.31 We have just started understanding the prognostic information inherent to ‘liquid biopsies’, while the methods by which CTCs and genetic markers should be monitored are still evolving and require close attention (Figure 1).31
Circulating melanoma cells
Human melanoma is the most malignant skin cancer with rapidly increasing incidence in industrialized countries worldwide. Although melanoma can be treated by surgical resection, metastatic melanoma is one of the most aggressive and drug-resistant neoplasm with poor OS.32,33 Current prognostic techniques are inadequate for disease management as many patients, considered clinically disease-free following primary tumour resection, later develop metastases. The 10-year survival rate for non-metastatic patients ranges from 39 to 93%, depending on primary tumour thickness, mitotic rate and presence of ulceration. There is no measure of residual disease in early-stage patients postsurgery; thus those requiring treatment cannot be identified. More sensitive procedures need to be developed in order to stage patients in a more accurate manner.34–36
It is now recognized that dissemination and implantation at distant sites of melanoma cells is a complex multi-step process influenced by both host and tumour characteristics.8,35,37,38 When cancer cells detach from the primary tumour, they can enter into blood or lymphatic system actively or passively. Passive intravasation is due to the detachment of CMCs from primary tumour as a consequence of increased haemodynamic flow.39–43 Recent biological data suggest that the active dislodgment of CMCs from the primary tumour is due to the acquisition of distinct modified phenotype and genotype that confer aggressiveness and metastatic efficiency.39 However, CMCs heterogeneity has a crucial role by allowing these cells to survive in the circulation and metastasize.39 Melanoma progression comprises transition from radial to vertical growth phase, epithelial-to-mesenchymal transition (EMT), alterations in cell adhesion properties and suppression of apoptosis.44,45 Precondition for metastasis is that surviving CMCs are able to adhere to endothelial surfaces and subsequently migrate into adjacent parenchyma in the target organ. More in detail, the key steps are: loss of adhesion, dermal invasion, migration from primary site, intravasation and subsequent survival in circulation, migration into distant tissues and subsequent proliferation, and enhancement of angiogenesis at the new colonized sites (Figure 2).33,39,46
E-CADH mediates homophilic cell–cell adhesion and has a crucial role in both epithelial cell–cell adhesion and in the maintenance of normal tissue architecture. Loss of E-CADH in normal melanocytes leads to the transition from benign lesions to invasive and metastatic cancer. It is considered as a tumour-suppressor gene acting as invasion-suppressor molecule: its loss permits and enhances invasion of adjacent normal tissues. Downregulation or complete shutdown of the E-CADH expression, mutation of the gene or other interference mechanisms involving the adherent junctions are strongly correlated with the loss of epithelial morphology and acquisition of metastatic potential.47 Once melanoma cells become invasive, they no longer express E-CADH but rather VE-CADH, (CDH5, Non-Epithelial Cadherin 5) or N-CADH (CDH2, Cadherin 2) both principally involved in EMT.47,48 Mel39 describes the process as follows: ‘the EMT process is used by migrating cells during embryonic development. It involves switching of polarized epithelial cells to contractile, motile mesenchymal progenitor cells, and is triggered by secretion of growth factors EGF (epithelial growth factor), FGF (fibroblast growth factor), and chemotactic/pro-migratory factors SF/HGF (hepatocyte growth factor) and chemokines from stromal fibroblasts and macrophages’.39
Because of EMT, melanocytic cells acquire the characteristics of their mesenchymal progenitor cells.46,49 Invasion is stimulated by increased activity of Wnt 5a and Notch pathways (increased expression of transcription factors Twist and Snail) and activation of PKC causing cytoskeletal changes that enhance cell motility.49,50 Twist, in particular, has an essential role in inhibiting E-CADH expression, inducing cell motility and contributing to metastasis by promoting EMT.51–53 In addition, E-CADH acts as an antiapoptotic factor enhancing cancer cell survival.54
Increasing evidence has suggested that phosphatase and tensin homolog (PTEN) is one of the powerful switches for the conversion between tumour suppressors and oncogenes. PTEN regulates a number of cellular processes, including cell growth, differentiation, cell death, proliferation, survival, motility, invasion and intracellular trafficking, through the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway. Furthermore, a number of studies have suggested that PTEN deletions may alter various functions of certain tumour-suppressor and oncogenic proteins. Alterations in this pathway, PTEN loss and/or AKT activation switches, mainly PTEN inactivation, have been associated with resistance to apoptosis and progression to tumorigenesis.49,55–58 Other proteins, involved in signalling pathways are enrolled in the evasion of apoptosis by CMCs, including a decrease in the expression of death receptors, increased expression of matrix metalloproteinases, increased secretion of growth factors and overexpression of antiapoptotic proteins (BCL-2, BCL-XL).57
It is renowned that such an aggressive tumour growth is strictly linked to the presence of blood vessels within and around tumour parenchyma59,60 as well as to the parallel dynamic expansion of the vasculature.61–63 The ability of tumour cells to recruit and maintain a ‘private’ vascular supply is achieved through several cellular processes involving interactions between tumour cells and their adjacent vascular endothelium, enhancing tumour growth.33 It is clear indeed that blood vessel formation process is complex and involves various interactions well exhaustively reviewed in literature.64 It is important to consider that cancer neovascularization must be triggered at a relatively early stage of tumour progression by a seemingly distinct event referred to as ‘the Angiogenic Switch’.61 This ‘Angiogenic Switch’ is a result of a change in balance between angiogenesis stimulators inhibitors and modulators present at the site of tumour growth.61,65 It seems to be influenced by genetic factors: more than 20 transforming oncogenes (that is, ras, myc, EGFR, HER-2), a large number of tumour-suppressor genes (that is, p53, PTEN) and epigenetic nature, such as hypoxia, inflammation and hormonal stimulation.65–72 Angiogenesis is the crucial event in melanoma metastatic progression and sustenance of cells within tumour.64
Detection of CMCs
Melanoma-associated antigen expression analysis
CMCs were studied for the first time in 1991 by detecting tyrosinase (Tyr-OH)—key enzyme of melanogenesis—in peripheral blood where the expression of this transcript is not normally expected.73–79
Various methods have been used to quantify and characterize CMCs in peripheral blood, without tumour cell separation, including indirect methods, such as reverse transcriptase-PCR (RT-PCR) and quantitative-RT PCR (qRT-PCR). Indirect methods are based on the assumption that, as melanocytes do not circulate, specific detection of melanocytic transcripts should correlate with CMCs.39,80,81 These cells are detectable in peripheral blood either soon after the surgical resection of primary tumours, regardless of their thickness, late-stage disease and even in clinically disease-free patients.76,82 These findings are confirmed by the percentage of positive cases for CMCs, ranging from 6 to 93% of the reports.75,77,78,83–85
RT-PCR is a variant of PCR. In RT-PCR, the RNA template is at first converted into a complementary DNA (cDNA) using a reverse transcriptase. cDNA is then used as a template to exponentially amplify target DNA sequences.86 Multiple markers RT-PCR assay has been established as the most reliable and sensitive approach to identify CMCs in peripheral blood or in draining lymph nodes of melanoma patients that express the putative transcripts of tumour-specific genes.74,75,77,79,85,87–89 In the past 10 years, many reports have focussed on the prognostic value of melanoma-associated-markers of differentiation (MAMs),75,90,91 such as Tyr-OH, MART-1 (Melan A/MLANA), B4GALNT1 (beta-1,4-N acetyl-galactosaminyl transferase 1), MAGE-3 (melanoma-antigen -family A3), p97 (melanotransferin/MFI2), MCAM (MUC 18/CD146, MelCAM), TRP-1/TRP-2 (tyrosinase-related protein 1 and 2), MSCP (melanoma chondroitin sulphate proteoglycan and ABCB5 (ATP-binding cassette transporter 5; Table 1).32,73–80,83,86,88,90–99 To date, different studies employed multi-marker assays to improve sensitivity and specificity of these procedures.74,75,77–79,83,88–92,95
Our research group also performed a highly specific and sensitive multi-marker RT-PCR assay focussed on Tyr-OH, MART-1, MAGE-3, MCAM/MUC18/CD146, MCAM and p97 expression levels.85
These MAMs were selected for their specificity and selectivity for melanoma cells, for the expression frequency in melanoma and for their high rate of detection in the RT-PCR assay.74,75,77–79,83,85,89–92,95,96,100 In a first study, we enrolled prospectively 100 patients (American Joint Committee on Cancer (AJCC) stages I–IV) affected by primary cutaneous melanoma or by distant metastases in cases of occult primary melanomas to analyse the co-expression of these markers in blood samples and confirm the reliability of the method.
We could validate that, among the investigated markers, Tyr-OH was the most frequent one in blood (15%), followed by MCAM/MUC18/CD146 (11%), MART-1 (5%), MAGE-3 (4%) and p97 (2%). In addition, Tyr-OH mRNA, alone or co-expressed with MART-1 or with p97, was not significantly associated with any AJCC stage. On the other hand, MART-1 and p97 were always co-expressed with Tyr-OH. MAGE-3 was detected alone or in combination with other MAMs without any significant association with AJCC stage. Our data do not confirm the report by Reynolds et al.90 that found MAGE-3 less frequently in advanced than in early stages. MAMs investigated in our series, namely, Tyr-OH, MART-, MAGE-3 and p97, resulted as not statistically related to any particular AJCC stage in contrast with the data reported on melanoma cell lines. Obviously, detection of melanoma biomarkers is hampered by the paucity of tumour material available in blood-based experiments, compared with in vitro growth cell lines.
Multi-marker RT-PCR assay of MAMs resulted as not so representative of the in vivo conditions and may give false interpretations of the gene expression. Loss of antigens—a possible event during disease progression—could occur and characterize CMCs, thus explaining our observation.
We highlighted that, when we reanalysed the patients expressing at least once MCAM/MUC18/CD146 either alone or co-expressed with other MAMs, it maintained its statistical significance with advanced stages.85,101
More recently, several groups developed multi-marker qRT-PCR, showing that levels of gene expression are associated with CMCs in patient blood and correlate to AJCC stage, survival and disease recurrences.34,39,80,85,102 In particular, qRT-PCR assays show an accurate and less laborious approach to molecular diagnosis, allowing a rapid and reproducible analysis.
Reid et al.103 in 2013 developed a multi-marker qRT-PCR for some melanocytic marker tumour-associated genes such as MCAM/MUC18/CD146, PAX3d, MART-1/MLANA, TGFβ and a stem cell marker ABCB5. They documented that melanoma patients expressed significantly one (92 versus 59%) or more markers (86 versus 17%) than healthy donor controls. In particular, MART-1/MLANA and ABCB5 are more likely expressed in advanced AJCC stages (III–IV) and co-expression or initial expression of these markers seem to be associated with higher risk of recurrence. MCAM/MUC18/CD146 was significantly more common in non-surgically treated advanced melanoma patients with a negative outcome than in those with a positive outcome (43 versus 9%), reasonably due to an ineffective eradication of CMCs.
They then concluded that MART-1/ MLANA and ABCB5 are helpful in following high-risk melanoma patients confirming that MCAM/MUC18/CD146 expression is associated with poorer outcome in stage IV disease. Recently, Venditelli et al.97 improved the qRT-PCR multi-marker assay by analysing six markers, including also the markers already described by Reid et al.49,103–105 They highlighted the co-expression of PAX3d, TGFβ and MTIFm without confirming the data by Reid et al.103 about the role of ABCB5 and MCAM/MUC18/CD146.
As reported by Mel,39 ‘a plethora of studies have focussed on identifying markers with sufficient specificity to accurately predict melanoma progression. Although many of these markers were identified using primary tissue or melanoma cell lines, they have been used for the multitude of CMC studies conducted until now'.74,75,77–79,83,85,89–92,95,96,100
Although molecular monitoring of whole-blood melanoma circulating RNA transcripts is still under debate, additional markers are required in order to understand better the diagnostic and prognostic significance of CMCs. In particular, the ability to isolate and measure CMCs represents a potentially powerful method to monitor patients with minimal morbidity and—more specifically—for an accurate diagnosis and prognosis.
Selection, isolation and enrichment of CMCs
The rarity of CMCs in the blood stream (1–3 CMCs/~5 billion blood cells) and the lack of a standardized technology to isolate every CMC will require further efforts and technological advancements. Therefore, specific isolation and enrichment of CMC population from a clinical sample is necessary before proceeding with biological and genetic characterization of these cells. Once collected, CMCs can be subjected to molecular biology, immuno-cytochemical and cytometric approaches. Isolation of CMC based on cell size-capture technology or cell density such as filtration, density gradient centrifugation and separation from red blood cells or leukocytes requires close attention. Effectively, these strategies, which isolate CMCs and avoid the leukocyte contamination, have suggested that these cells may span a wide range of cell sizes.106,107 Also CMCs' isolation by analysing marker protein antigens seems complicated by the heterogeneity of the cell surface. Furthermore, CMC can acquire new genetic mutations from the tumour of origin and can be induced to phenotypic drift. This contributes to cellular heterogeneity accounting for the high variation among markers expressed by these cells.108–111 Some recent studies examined cytokines and phenotype profiles of freshly patient-derived human skin melanoma cells or freshly surgically excised lymph node melanoma. A great number of antigens was identified that now require validation in a larger cohort of patients and need to be explained.
In tumours as carcinomas, immuno-magnetic enrichment is the most commonly used technique for CTC collection. Immuno-magnetic antibodies against EpCAM have been used to target CTCs, followed by magnetic separation and optical analysis to isolate and reliably detect CTCs (CellSearch Circulating Tumor Cell, Janssen Diagnostic, LLC, Raritan, NJ, USA). The method that uses an antigen expressed by the tumour cells as a means to capture and isolate the aforementioned cells is referred to as ‘positive’ selection. Melanoma does not express the classical epithelial cell surface marker EpCAM, which has formed its basis for most CTC isolation,112 and is the only Food and Drug Administration-approved platform for the prognosis of this kind of carcinoma. Melanocytes origin from neural crest and have been associated with some specific melanoma cell surface epitopes, such as MCAM/MUC18/CD146 and MSCP/NG2 (melanoma-associated Chondroitin Sulphate) and with stem cell markers, such as ABCB5 (ATP-binging cassette subfamily member B) and CD 271.20,32,88,96,97,113–115
Indeed, given the difficulty of isolating whole CMCs, MCAM/MUC18/MelCAM/CD146 has been proposed as a substitute for EpCAM in immuno-targeting and separation of melanoma cells using positive selection. An alternative to the ‘positive selection’ CMC strategy is the ‘negative’ selection in which cells of interest are enriched through the depletion of unwanted cells. Negative selection is good for isolating cells with poorly characterized immuno-phenotype, such as CMCs. Figure 2 shows the main current methods for the detection of CTCs.
The presence of CTCs was identified as an independent prognostic marker in a number of metastatic cancers.17–22,114 The number of CTCs has been shown as indicative of the length of PFS and OS.18,23,24 For melanoma, few studies have explained in detail the prognostic value of CMCs. Two studies20,114 have shown that the number of CMCs is prognostic of OS, with >2 CMCs per 7.5 ml of blood associated with shorter survival. Both studies have been performed by using the Cell-Search Melanoma Kit (Veridex platform), which identifies double MCAM/MUC18/CD146-MCSP positive, double CD45-CD34 negative cells as CMCs.18,23,24,116 In particular, Khoja et al.20 indicated that 26% of metastatic melanoma patients have 2× CMCs at baseline and that median OS was shorter than those with <2 CMCs (2.6 versus 7.2 months, log-rank P<0.009). The employ of multi-marker approach has shown that the decrease in CMCs after therapy initiation is associated with response to treatment and prolonged OS in vemurafenib (anti-BRAFV600E mutation targeted therapy) treated patients. It should be noted that, the cutoff of 2 CMCs per 7.5 ml of blood is an independent prognostic biomarker. In contrast with this last data, Klinac et al.17 did not define a baseline CMC number (prior to treatment) as prognostic of OS or disease -free-survival.17 However, the level of circulating antigen-expressing tumour cells is still unknown, mostly due to the limited possibility to use the current technologies: CMCs are detectable in only 40% of patients with advanced melanoma. Furthermore, CMC characterization with additional markers would be added to the standard Cell-Search Melanoma Kit (ABCB5, CD 271)17 improving molecular subtyping and possibly tailoring the treatments.
MCAM/MUC18/CD146/MelCAM/CD146
Prognostic biomarker and innovative antitumoural strategy in melanoma
MCAM/MUC18/CD146, a melanoma cell adhesion molecule, is recently obtaining more attention as a novel biomarker for disease progression and poor outcome in patients affected by melanoma.117–119 Also cited as CD146, A32 antigen or S-Endo-1, it belongs to the immunoglobulin superfamily being mainly expressed at the intercellular junction of endothelial cells where it interacts directly with VEGFR-2.98,120,121 Originally identified in melanoma but not in normal tissue, it is now being investigated in development, signal transduction, cell migration, mesenchymal stem cells differentiation, angiogenesis and immune response.99 Many reports indicate that MCAM/MUC18/CD146 correlates with tumour thickness and metastatic potential of human melanoma cells in mice and in humans.99,122–127 As an endothelial antigen, MCAM/MUC18/CD146 affects angiogenesis promoting neoplastic progression from local invasive to metastatic disease by upregulating MMP-2 metalloproteinase and through cell interaction among extracellular matrix and vascular endothelial.128 Mills et al.129 studied the effect of a fully humanized anti-MCAM/MUC18/CD146 antibody (ABX-MA1) on tumour growth, angiogenesis and metastasis of human melanoma. ABX-MA1 treatment of melanoma cells was able to inhibit the promoter and collagenase activity of MMP-2 (Figures 3 and 4). Reduced MMP-2 expression was observed in implanted tumours in vivo.129,130
Moreover, as already described above, we reported that MCAM/MCAM/MUC18/CD146—either alone or co-expressed with other MAMs—maintained its statistical significance with advanced stages. The presence of MCAM/MUC18/CD146 increases the possibility of being in advanced AJCC III–IV stages and have a higher incidence of recurrences.86,131
All these findings strongly support a reliable role of MCAM/MUC18/CD146 in melanoma progression. Thus we decided to extend our analysis to a larger series of patients exploring circulating MCAM/MUC18/CD146 expression by RT-PCR assay on serial blood samples obtained during the clinical course of the disease.
Our investigation132 emphasized a correspondence among MCAM/MUC18/CD146 mRNA blood level, detection and degree of expression of this marker on the corresponding primary melanoma tissue, tumour thickness, AJCC stages and clinical outcome. We showed that MCAM/MUC18/CD146 RT-PCR assay for CMCs correlated with melanoma diagnosis and progression of the disease. Either if already detectable from the beginning or subsequently acquired during the course of the disease, MCAM/MUC18/CD146 was significantly associated with poor prognosis and death.
Differently from Reid et al.,103 we detected MCAM/MUC18/CD14 even in early AJCC stages, but surprisingly, the patients who lost this marker are still clinically disease free. On the contrary, patients affected at an early stage (AJCC stage IIB) who later acquired a persisting MCAM/MUC18/CD146 status, unfortunately then suffered from disease progression. The comparison of the clinical outcome between early AJCC stages patients sharing fleeting expression, and patients who later acquired a persisting expression, resulted statistically significant. Considering patients in advanced stages, we emphasized a statistically significant difference comparing clinical course and outcome of MCAM/MUC18/CD146-positive patients to those who never expressed this biomarker, with good outcome or stable disease. Immune surveillance or haemodynamic forces can somehow limit the different behaviour and progression.133 Therefore, transient CMCs expressing MCAM/MUC18/CD146—either related to the tumour burden or spread after the surgical excision—should be interpreted as limited survival early micrometastases with short half-life and consequent absence of clinical proliferating activity. While a persisting or later achieved MCAM/MUC18/CD146 detection could indicate a mature metastatic proliferative behaviour, capable of spreading into the surrounding tissue through the degrading basement membrane and extracellular matrix.3,6,133
In contrast with the data of Reid et al.,103 Venditelli et al.97 do not confirm a significant correlation of MCAM/MUC18/CD146 and ABCB5 transcripts with both Breslow classes and staging: ABCB5 does not represent a useful molecular marker for melanoma diagnosis or for melanoma targeted therapy. Moreover, in contrast with our data,131,132 they describe high MCAM baseline expression due to its endothelial component, constitutively expressed. Regarding this aspect, we have to focus on the two MCAM/MUC18/CD146 isoforms,134,135 a Long and a Short variant, different from each other for the 3’ cytoplasmic tail that may or not contain 34 amino-acid residues encoded by exon 15. This alternative splicing generates the Short isoform by direct junction of exon 14 to exon 16, with the excision of exon 15. The Short isoform is widely expressed by endothelial cells, rather than the Long isoform that seems expressed preferentially by melanoma primary culture cells. An interesting commentary on the publication of Reid et al.103 has been previously reported.136 The authors underline that MCAM transcripts, analysed by qRT-PCR, may fluctuate significantly in the healthy population. They suppose that the elevated copy number, also present in normal individuals, could be related to one of the two MCAM/MUC18/CD146 isoforms. Therefore, they hypothesize a possible MCAM transcript overestimation mainly due to Short isoform detection. They explain this feature by considering intercellular interactions between melanoma cells and vascular endothelium, particularly during metastatic process. Furthermore, they suggest a particularly accurate design of primers and probes in order to minimize the effect of endothelial contamination.136 According to this last consideration, we describe our preliminary pilot study performed on CMCs isolated and enriched from patients affected by melanoma at least by AJCC stage Ib, by targeting the MCAM/MUC18/CD146 antigen with immune-magnetic beads coated with antibody against MCAM/MUC18/CD146 antigen. The expression study of Long, Short isoforms and extracellular domain of MCAM/MUC18/CD146, performed by home-made designing specific primers, nicely documented the co-expression of both isoforms on enriched cells isolated from melanoma patients with respect to healthy subjects only carrying the Short variant. In our view, the most interesting emerging data are obtained by using primers mapping at the 5’ upstream of the transcript (NM_006500-3332 bp).74 Effectively, the persistent expression or achievement of this specific molecular transcript seems to characterize advanced melanoma status, also analysing selected CD146-positive cells. The transcript, corresponding to the first extracellular domain of the antigen, is not detected in circulating enriched cells selected from healthy donors, even if expressing the Short isoform. Obviously, an accurate CMC enrichment need to be assessed and these preliminary data need to be confirmed. The group of Blot-Chabaud137,138 identified, in addition to the membrane-anchored form of MCAM/MUC18/CD146, a soluble form of CD146 (sCD146/MCAM/MUC18),139–141 which is mainly generated by the proteolytic cleavage of the membrane form through metalloproteases.141 Interestingly, the sCD146/MCAM/MUC18 concentrations increased in several diseases, in particular in tumours.142 Moreover, sCD146/MCAM/MUC18 constitutes an active factor having a major role in angiogenesis.143 They documented that this effect was mediated through the binding of sCD146/MCAM/MUC18 on the p80 isoform of angiomotin.120 This protein is not only detectable on the vasculature of ischemic tissues143 but also on many tumour cells.144–149 These findings suggest that MCAM/MUC18/CD146-positive tumours could secrete soluble CD146 that, in turn, would be responsible for their growth and vascularization. In particular, in this study, sCD146/MCAM/MUC18 secreted by CD146-positive tumours does not only display effects on tumour angiogenesis but also on tumour growth and survival. Thus sCD146/MCAM/MUC18 induces the expression of either proteins involved in tumour proliferation and invasion or proteins inhibiting apoptosis and senescence. The decision to focus the attention on the circulating form of the protein could be extremely powerful to target sCD146/MCAM/MUC18 with monoclonal antibody capable of neutralizing its effects without affecting the membrane MCAM/MUC18/CD146. This approach could constitute an innovative antitumoural strategy. These data demonstrate that the active powerful action on angiogenesis, tumour growth and disease progression, is exerted by the extracellular portion of the protein. Taken together, MCAM/MUC18/CD146 molecular expression analysis, sequential monitoring of the transcripts and detection of the soluble form could help to investigate or follow the melanoma remission or progression even in apparent disease-free status. MCAM/MUC18/CD146 behaves as a ‘molecular warning of progression’ and its targeting could constitute an innovative therapeutic strategy for the CD146-positive melanoma.
References
Fidler IJ, Ellis LM . The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994; 79: 185–188.
Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD . The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 2002; 3: 53–57.
Paterlini-Brechot P, Benali NL . Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 2007; 253: 180–204.
Weiss L . Metastatic inefficiency. Adv Cancer Res 1990; 54: 159–211.
Fidler IJ . Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst 1970; 45: 773–782.
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998; 153: 865–873.
Mocellin S, Keilholz U, Rossi CR, Nitti D . Circulating tumor cells: the 'leukemic phase' of solid cancers. Trends Mol Med 2006; 12: 130–139.
Jacob K, Sollier C, Jabado N . Circulating tumor cells: detection, molecular profiling and future prospects. Expert Rev Proteomics 2007; 4: 741–756.
Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol 2007; 25: 5194–5202.
Lembessis P, Msaouel P, Halapas A, Sourla A, Panteleakou Z, Pissimissis N et al. Combined androgen blockade therapy can convert RT-PCR detection of prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA) transcripts from positive to negative in the peripheral blood of patients with clinically localized prostate cancer and increase biochemical failure-free survival after curative therapy. Clin Chem Lab Med 2007; 45: 1488–1494.
Tsouma A, Aggeli C, Pissimissis N, Lembessis P, Zografos GN, Koutsilieris M . Circulating tumor cells in colorectal cancer: detection methods and clinical significance. Anticancer Res 2008; 28: 3945–3960.
Panteleakou Z, Lembessis P, Sourla A, Pissimissis N, Polyzos A, Deliveliotis C et al. Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Mol Med 2009; 15: 101–114.
Mitsiades CS, Lembessis P, Sourla A, Milathianakis C, Tsintavis A, Koutsilieris M . Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin Exp Metastasis 2004; 21: 495–505.
Ross JS, Slodkowska EA . Circulating and disseminated tumor cells in the management of breast cancer. Am J Clin Pathol 2009; 132: 237–245.
Theodoropoulos PA, Polioudaki H, Agelaki S, Kallergi G, Saridaki Z, Mavroudis D et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett 2010; 288: 99–106.
Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res 2008; 14: 2593–2600.
Klinac D, Gray ES, Freeman JB, Reid A, Bowyer S, Millward M et al. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 2014; 14: 423.
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14: 6302–6309.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012; 30: 525–532.
Khoja L, Lorigan P, Zhou C, Lancashire M, Booth J, Cummings J et al. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J Invest Dermatol 2013; 133: 1582–1590.
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29: 1556–1563.
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007; 450: 1235–1239.
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 3213–3221.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791.
Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009; 175: 808–816.
Hartkopf AD, Wagner P, Wallwiener D, Fehm T, Rothmund R . Changing levels of circulating tumor cells in monitoring chemotherapy response in patients with metastatic breast cancer. Anticancer Res 2011; 31: 979–984.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006; 12: 4218–4224.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006; 12: 6403–6409.
Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Murphy GF et al. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun 2010; 402: 711–717.
Alix-Panabieres C, Pantel K . Circulating tumour cells: liquid biopsy of cancer. Clin Chem 2013; 59: 110–118.
Pantel K, Alix-Panabieres C . Liquid biopsy: potential and challenges. Mol Oncol 2016; 10: 371–373.
Schatton T, Frank MH . Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 2008; 21: 39–55.
Nezos A, Msaouel P, Pissimissis N, Lembessis P, Sourla A, Armakolas A et al. Methods of detection of circulating melanoma cells: a comparative overview. Cancer Treat Rev 2011; 37: 284–290.
Koyanagi K, Kuo C, Nakagawa T, Mori T, Ueno H, Lorico AR et al. Multimarker quantitative real-time PCR detection of circulating melanoma cells in peripheral blood: relation to disease stage in melanoma patients. Clin Chem 2005; 51: 981–988.
Palmieri G, Casula M, Sini MC, Ascierto PA, Cossu A . Issues affecting molecular staging in the management of patients with melanoma. J Cell Mol Med 2007; 11: 1052–1068.
Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Goydos JS, Beitsch PD et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol 2006; 24: 2849–2857.
Miller AJ, Mihm MC Jr . Melanoma. N Engl J Med 2006; 355: 51–65.
Chin L . The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer 2003; 3: 559–570.
Mel Z . Circulating melanoma cells. In: Tanaka Y (ed). Breakthroughs in Melanoma Research, Chapter 3. INTECH open science-open minds, 2011.
Bockhorn M, Jain RK, Munn LL . Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 2007; 8: 444–448.
Chen LL, Blumm N, Christakis NA, Barabasi AL, Deisboeck TS . Cancer metastasis networks and the prediction of progression patterns. Br J Cancer 2009; 101: 749–758.
Dvorak HF . Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659.
Liang S, Slattery MJ, Wagner D, Simon SI, Dong C . Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng 2008; 36: 661–671.
Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res 2006; 19: 290–302.
Mandruzzato S, Callegaro A, Turcatel G, Francescato S, Montesco MC, Chiarion-Sileni V et al. A gene expression signature associated with survival in metastatic melanoma. J Transl Med 2006; 4: 50.
Mazzocca A, Carloni V . The metastatic process: methodological advances and pharmacological challenges. Curr Med Chem 2009; 16: 1704–1717.
Pecina-Slaus N . Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 2003; 3: 17.
Moustakas A, Heldin CH . Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 2007; 98: 1512–1520.
Mumford BS, Robertson GP . Circulating melanoma cells in the diagnosis and monitoring of melanoma: an appraisal of clinical potential. Mol Diagn Ther 2014; 18: 175–183.
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002; 1: 279–288.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939.
Thisse B, el Messal M, Perrin-Schmitt F . The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res 1987; 15: 3439–3453.
Niu RF, Zhang L, Xi GM, Wei XY, Yang Y, Shi YR et al. Up-regulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res 2007; 26: 385–394.
Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev 1999; 13: 2207–2217.
Robertson GP . Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev 2005; 24: 273–285.
Madhunapantula SV, Sharma A, Robertson GP . PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res 2007; 67: 3626–3636.
Mehlen P, Puisieux A . Metastasis: a question of life or death. Nat Rev Cancer 2006; 6: 449–458.
Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP . Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res 2003; 63: 2881–2890.
Yu JL, Rak JW, Carmeliet P, Nagy A, Kerbel RS, Coomber BL . Heterogeneous vascular dependence of tumor cell populations. Am J Pathol 2001; 158: 1325–1334.
Coomber BL, Yu JL, Fathers KE, Plumb C, Rak JW . Angiogenesis and the role of epigenetics in metastasis. Clin Exp Metastasis 2003; 20: 215–227.
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31.
Folkman J . Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333: 1757–1763.
Folkman J . Angiogenesis-dependent diseases. Semin Oncol 2001; 28: 536–542.
Carmeliet P, Jain RK . Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257.
Rak J, Yu JL, Klement G, Kerbel RS . Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J Investig Dermatol Symp Proc 2000; 5: 24–33.
Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ . Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res 2006; 66: 4249–4255.
Bouck N, Stellmach V, Hsu SC . How tumors become angiogenic. Adv Cancer Res 1996; 69: 135–174.
Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999; 401: 670–677.
Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ . Genetic heterogeneity of angiogenesis in mice. FASEB J 2000; 14: 871–876.
Harris AL . Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Semenza GL . Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 2000; 35: 71–103.
Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci USA 1998; 95: 10820–10825.
Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE . Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet 1991; 338: 1227–1229.
Hoon DS, Wang Y, Dale PS, Conrad AJ, Schmid P, Garrison D et al. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol 1995; 13: 2109–2116.
Curry BJ, Myers K, Hersey P . Polymerase chain reaction detection of melanoma cells in the circulation: relation to clinical stage, surgical treatment, and recurrence from melanoma. J Clin Oncol 1998; 16: 1760–1769.
Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med 1994; 179: 921–930.
Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ . A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J 1992; 11: 519–526.
Mellado B, Gutierrez L, Castel T, Colomer D, Fontanillas M, Castro J et al. Prognostic significance of the detection of circulating malignant cells by reverse transcriptase-polymerase chain reaction in long-term clinically disease-free melanoma patients. Clin Cancer Res 1999; 5: 1843–1848.
Palmieri G, Strazzullo M, Ascierto PA, Satriano SM, Daponte A, Castello G . Polymerase chain reaction-based detection of circulating melanoma cells as an effective marker of tumor progression. Melanoma Cooperative Group. J Clin Oncol 1999; 17: 304–311.
Rose TM, Plowman GD, Teplow DB, Dreyer WJ, Hellström KE, Brown JP . Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc Natl Acad Sci USA 1986; 83: 1261–1265.
Keilholz U, Goldin-Lang P, Bechrakis NE, Max N, Letsch A, Schmittel A et al. Quantitative detection of circulating tumor cells in cutaneous and ocular melanoma and quality assessment by real-time reverse transcriptase-polymerase chain reaction. Clin Cancer Res 2004; 10: 1605–1612.
Ghossein RA, Rosai J . Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer 1996; 78: 10–16.
Schittek B, Bodingbauer Y, Ellwanger U, Blaheta HJ, Garbe C . Amplification of MelanA messenger RNA in addition to tyrosinase increases sensitivity of melanoma cell detection in peripheral blood and is associated with the clinical stage and prognosis of malignant melanoma. Br J Dermatol 1999; 141: 30–36.
Hoon DS, Bostick P, Kuo C, Okamoto T, Wang HJ, Elashoff R et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res 2000; 60: 2253–2257.
Rapanotti MC, Bianchi L, Ricozzi I, Campione E, Pierantozzi A, Orlandi A et al. Melanoma-associated markers expression in blood: MUC-18 is associated with advanced stages in melanoma patients. Br J Dermatol 2009; 160: 338–344.
Kuo CT, Bostick PJ, Irie RF, Morton DL, Conrad AJ, Hoon DS . Assessment of messenger RNA of beta 1-->4-N-acetylgalactosaminyl-transferase as a molecular marker for metastatic melanoma. Clin Cancer Res 1998; 4: 411–418.
Orlow SJ, Hearing VJ, Sakai C, Urabe K, Zhou BK, Silvers WK et al. Changes in expression of putative antigens encoded by pigment genes in mouse melanomas at different stages of malignant progression. Proc Natl Acad Sci USA 1995; 92: 10152–10156.
Yang J, Price MA, Li GY, Bar-Eli M, Salgia R, Jagedeeswaran R et al. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res 2009; 69: 7538–7547.
Wascher RA, Morton DL, Kuo C, Elashoff RM, Wang HJ, Gerami M et al. Molecular tumor markers in the blood: early prediction of disease outcome in melanoma patients treated with a melanoma vaccine. J Clin Oncol 2003; 21: 2558–2563.
Reynolds SR, Albrecht J, Shapiro RL, Roses DF, Harris MN, Conrad A et al. Changes in the presence of multiple markers of circulating melanoma cells correlate with clinical outcome in patients with melanoma. Clin Cancer Res 2003; 9: 1497–1502.
Jiménez-Cervantes C, Solano F, Lozano JA, Garcia-Borrón JC . Tyrosinase isoenzymes: two melanosomal tyrosinases with different kinetic properties and susceptibility to inhibition by calcium. Pigment Cell Res 1994; 7: 291–297.
Aubin F, Chtourou M, Teyssier JR, Laubriet A, Mougin CH, Blanc D et al. The detection of tyrosinase mRNA in the peripheral blood of stage I melanoma patients is not of clinical relevance in predicting metastasis risk and survival. Melanoma Res 2000; 10: 113–118.
Sarantou T, Chi DD, Garrison DA, Conrad AJ, Schmid P, Morton DL, Hoon DS . Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res 1997; 57: 1371–1376.
Kitago M, Koyanagi K, Nakamura T, Goto Y, Faries M, O'Day SJ et al. mRNA expression and BRAF mutation in circulating melanoma cells isolated from peripheral blood with high molecular weight melanoma-associated antigen-specific monoclonal antibody beads. Clin Chem 2009; 55: 757–764.
Brownbridge GG, Gold J, Edward M, MacKie RM . Evaluation of the use of tyrosinase-specific and melanA/MART-1-specific reverse transcriptase-coupled–polymerase chain reaction to detect melanoma cells in peripheral blood samples from 299 patients with malignant melanoma. Br J Dermatol 2001; 144: 279–287.
Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003; 278: 47156–47165.
Vendittelli F, Paolillo C, Autilio C, Lavieri MM, Silveri SL, Capizzi R et al. Absolute quantitative PCR for detection of molecular biomarkers in melanoma patients: a preliminary report. Clin Chim Acta 2015; 444: 242–249.
Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmuller G, Johnson JP . Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113 000 and a protein with a molecular weight of 76 000. Cancer Res 1987; 47: 841–845.
Melnikova VO, Bar-Eli M . Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res 2006; 19: 395–405.
Gogas H, Eggermont AM, Hauschild A, Hersey P, Mohr P, Schadendorf D et al. Biomarkers in melanoma. Ann Oncol 2009; 20(Suppl 6): 8–13.
Rapanotti MC, Ricozzi I, Campione E, Orlandi A, Bianchi L . Blood MUC-18/MCAM expression in patients with melanoma: a suitable marker of poor outcome. Br J Dermatol 2013; 169: 221–222.
Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR . The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res 2006; 12: 4605–4613.
Reid AL, Millward M, Pearce R, Lee M, Frank MH, Ireland A et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol 2013; 168: 85–92.
Parker CJ, Shawcross SG, Li H, Wang QY, Herrington CS, Kumar S et al. Expression of PAX 3 alternatively spliced transcripts and identification of two new isoforms in human tumors of neural crest origin. Int J Cancer 2004; 108: 314–320.
Matsuzaki Y, Hashimoto S, Fujita T, Suzuki T, Sakurai T, Matsushima K et al. Systematic identification of human melanoma antigens using serial analysis of gene expression (SAGE). J Immunother 2005; 28: 10–19.
De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 2010; 130: 2440–2447.
Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013; 5: 174–179.
Welch DR, Tomasovic SP . Implications of tumor progression on clinical oncology. Clin Exp Metastasis 1985; 3: 151–188.
Riethdorf S, Pantel K . Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann NY Acad Sci 2010; 1210: 66–77.
Klein CA, Blankenstein TJ, Schmidt-Kittler O, Petronio M, Polzer B, Stoecklein NH et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet 2002; 360: 683–689.
Strauss BS . Hypermutability and silent mutations in human carcinogenesis. Semin Cancer Biol 1998; 8: 431–438.
Yu M, Stott S, Toner M, Maheswaran S, Haber DA . Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 2011; 192: 373–382.
Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010; 466: 133–137.
Rao C, Bui T, Connelly M, Doyle G, Karydis I, Middleton MR et al. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol 2011; 38: 755–760.
Luo X, Mitra D, Sullivan RJ, Wittner BS, Kimura AM, Pan S et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep 2014; 7: 645–653.
Freeman JB, Gray ES, Millward M, Pearce R, Ziman M . Evaluation of a multi-marker immunomagnetic enrichment assay for the quantification of circulating melanoma cells. J Transl Med 2012; 10: 192.
Shih IM . The role of CD146 (Mel-CAM) in biology and pathology. J Pathol 1999; 189: 4–11.
Mobley AK, Braeuer RR, Kamiya T, Shoshan E, Bar-Eli M . Driving transcriptional regulators in melanoma metastasis. Cancer Metastasis Rev 2012; 31: 621–632.
Braeuer RR, Watson IR, Wu CJ, Mobley AK, Kamiya T, Shoshan E et al. Why is melanoma so metastatic? Pigment Cell Melanoma Res 2014; 27: 19–36.
Stalin J, Harhouri K, Hubert L, Subrini C, Lafitte D, Lissitzky JC et al. Soluble melanoma cell adhesion molecule (smcam/scd146) promotes angiogenic effects on endothelial progenitor cells through angiomotin. J Bio Chem 2013; 288: 8991–9000.
Shih LM, Hsu MY, Palazzo JP, Herlyn M . The cell-cell adhesion receptor mel-cam acts as a tumor suppressor in breast carcinoma. Am J Pathol 1997; 151: 745–751.
Holzmann B, Brocker EB, Lehmann JM, Ruiter DJ, Sorg C, Riethmuller G et al. Tumor progression in human malignant melanoma: Five stages defined by their antigenic phenotypes. Int J Cancer 1987; 39: 466–471.
Lehmann JM, Riethmuller G, Johnson JP . Muc18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA 1989; 86: 9891–9895.
Luca M, Hunt B, Bucana CD, Johnson JP, Fidler IJ, Bar-Eli M . Direct correlation between muc18 expression and metastatic potential of human melanoma cells. Melanoma Res 1993; 3: 35–41.
Xie S, Luca M, Huang S, Gutman M, Reich R, Johnson JP et al. Expression of mcam/muc18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res 1997; 57: 2295–2303.
Satyamoorthy K, Muyrers J, Meier F, Patel D, Herlyn M . Mel-CAM-specific genetic suppressor elements inhibit melanoma growth and invasion through loss of gap junctional communication. Oncogene 2001; 20: 4676–4684.
Haass NK, Smalley KS, Li L, Herlyn M . Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res 2005; 18: 150–159.
Zhang Y, Zheng C, Zhang J, Yang D, Feng J, Lu D et al. Generation and characterization of a panel of monoclonal antibodies against distinct epitopes of human CD146. Hybridoma (Larchmt) 2008; 27: 345–352.
Mills L, Tellez C, Huang S, Baker C, McCarty M, Green L et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res 2002; 62: 5106–5114.
McGary EC, Lev DC, Bar-Eli M . Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol Ther 2002; 1: 459–465.
Rapanotti M, Ricozzi I, Campione E, Orlandi A, Bianchi L . Blood muc-18/mcam expression in melanoma patients: A suitable marker of poor outcome. Br J Dermatol 2013; 169: 221–222.
Rapanotti MC, Suarez Viguria TM, Costanza G, Ricozzi I, Pierantozzi A, Di Stefani A et al. Sequential molecular analysis of circulating MCAM/MUC18 expression: a promising disease biomarker related to clinical outcome in melanoma. Arch Dermatol Res 2014; 306: 527–537.
Larson AR, Konat E, Alani RM . Melanoma biomarkers: current status and vision for the future. Nat Clin Pract Oncol 2009; 6: 105–117.
Vainio O, Dunon D, Aissi F, Dangy JP, McNagny KM, Imhof BA . HEMCAM, an adhesion molecule expressed by c-kit+ hemopoietic progenitors. J Cell Biol 1996; 135: 1655–1668.
Alais S, Allioli N, Pujades C, Duband JL, Vainio O, Imhof B et al. HEMCAM/CD146 downregulates cell surface expression of beta1 integrins. J Cell Sci 2001; 114: 1847–1859.
Capoluongo E, Paolillo C, Vendittelli F . Is quantitative real time polymerase chain reaction MCAM transcript assay really suitable for prognostic and predictive management of melanoma patients? Br J Dermatol 2001; 171: 190–191.
Harhouri K, Kebir A, Guillet B, Foucault-Bertaud A, Voytenko S, Piercecchi-Marti MD et al. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind-limb ischemia. Blood 2010; 115: 3843–3851.
Stalin J, Nollet M, Garigue P, Fernandez S, Vivancos L, Essaadi A et al. Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors. Oncogene 2016; 5: 1–12.
Bardin N, Frances V, Combes V, Sampol J, Dignat-George F . CD146: biosynthesis and production of a soluble form in human cultured endothelial cells. FEBS Lett 1998; 421: 12–14.
Bardin N, Moal V, Anfosso F, Daniel L, Brunet P, Sampol J et al. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb Haemost 2003; 90: 915–920.
Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 2009; 29: 746–753.
Ilie M, Long E, Hofman V, Selva E, Bonnetaud C, Boyer J et al. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br J Cancer 2014; 110: 1236–1243.
Stalin J, Harhouri K, Hubert L, Subrini C, Lafitte D, Lissitzky JC et al. Soluble melanoma cell adhesion molecule (sMCAM/sCD146) promotes angiogenic effects on endothelial progenitor cells through angiomotin. J Biol Chem 2013; 288: 8991–9000.
Roudier E, Chapados N, Decary S, Gineste C, Le Bel C, Lavoie JM et al. Angiomotin p80/p130 ratio: a new indicator of exercise-induced angiogenic activity in skeletal muscles from obese and non-obese rats? J Physiol 2009; 587: 4105–4119.
Jiang WG, Watkins G, Douglas-Jones A, Holmgren L, Mansel RE . Angiomotin and angiomotin like proteins, their expression and correlation with angiogenesis and clinical outcome in human breast cancer. BMC Cancer 2006; 6: 16–20.
Satchi-Fainaro R, Ferber S, Segal E, Ma L, Dixit N, Ijaz A et al. Prospective identification of glioblastoma cells generating dormant tumors. PLoS One 2012; 7: e44395.
Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev 2011; 25: 51–63.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2011; 27: 6199–6206.
Liotta LA, Kohn E . Anoikis: cancer and the homeless cell. Nature 2004; 430: 973–974.
Acknowledgements
We thank Tara Mayte Suarez Viguria for excellent technical assistance. We also thank Chiara Tarquini, PhD for her kind assistance in the editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Rapanotti, M., Campione, E., Spallone, G. et al. Minimal residual disease in melanoma: circulating melanoma cells and predictive role of MCAM/MUC18/MelCAM/CD146. Cell Death Discov. 3, 17005 (2017). https://doi.org/10.1038/cddiscovery.2017.5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/cddiscovery.2017.5
This article is cited by
-
Applications of liquid biopsy in the Pharmacological Audit Trail for anticancer drug development
Nature Reviews Clinical Oncology (2021)
-
Characterization of novel neutralizing mouse monoclonal antibody JM1-24-3 developed against MUC18 in metastatic melanoma
Journal of Experimental & Clinical Cancer Research (2020)