Abstract
Acute kidney injury (AKI) is a common and severe clinical condition with a heavy healthy burden around the world. In spite of supportive therapies, the mortality associated with AKI remains high. Our limited understanding of the complex cell death mechanism in the process of AKI impedes the development of desirable therapeutics. Necroptosis is a recently identified novel form of cell death contributing to numerable diseases and tissue damages. Increasing evidence has suggested that necroptosis has an important role in the pathogenesis of various types of AKI. Therefore, we present here the signaling pathways and main regulators of necroptosis that are potential candidate for therapeutic strategies. Moreover, we emphasize on the potential role and corresponding mechanisms of necroptosis in AKI based on recent advances, and also discuss the possible therapeutic regimens based on manipulating necroptosis. Taken together, the progress in this field sheds new light into the prevention and management of AKI in clinical practice.
Similar content being viewed by others
Facts
-
Necroptosis is a kind of regulated necrosis, a novel form of cell death with morphologic features of necrosis but molecularly controlled.
-
Necroptosis can be widely triggered by death receptors, toll-like receptors, interferons (IFNs), and intracellular protein DNA-dependent activator of IFN regulatory factors in response to viruses. Various upstream signaling converge on mediator receptor-interacting protein kinase 3 and share the common executor mixed lineage kinase domain-like protein.
-
Necroptosis has been implicated in the pathogenesis of diverse types of AKI to different extents.
-
Blocking necroptotic pathways by pharmacological inhibitors or genetic manipulation alleviates renal injuries in vitro and in vivo, indicating a promising outlook in the management of AKI.
Open Questions
-
How to monitor necroptosis in vivo?
-
Does necroptosis relate to different stages of AKI? If yes, how?
-
What is the mechanism of cell-type-specific vulnerability to necroptosis? And what are the clinical implications of this selective sensitivity in AKI?
-
How does necroptosis interplay with inflammation specifically during AKI?
-
What is the relative contribution of necroptosis and other forms of regulatory cell death in AKI?
Cell death, serving as an essential process throughout life, has been extensively investigated for decades. Historically, cell death was classified as distinctive forms: apoptosis, autophagic cell death and necrosis.1 The term ‘apoptosis’, deriving from ancient Greek, has long been recognized synonymously as the programmed cell death, which is developmentally programmed and molecularly controlled. Characteristic morphologic changes of apoptosis include cell shrinkage, nuclear and cytoplasmic condensation, DNA fragmentation, and the formation of apoptosomes.2 Autophagic cell death is a newly defined, highly regulated cell death, which is characterized by the markers of autophagy pathway.3, 4 In contrast, necrosis was thought to be accidental cell death, and results in cellular swelling, breakdown of plasma membrane integrity and release of intracellular contents, all of which are absolutely distinct from programmed cell death.5 Cell death, no matter which specific form, has a key role in maintaining tissue homeostasis as well as other crucial biological processes.
However, the full extent of cell death has not been explored. Recent breakthroughs in this field shed light on regulated necrosis, a new type of cell death with morphologic features of necrosis but genetically determined.6 It has been well-documented that necroptosis is highly orchestrated regulated necrosis dependent on receptor-interacting protein kinase 3 (RIP3) and mixed lineage kinase domain-like protein (MLKL).7, 8 It not only involves in the development of organism but also participates in various pathophysiological processes. An accumulating body of evidence has demonstrated that necroptosis contributes to the pathogenesis of numerous diseases and tissue damages, including ischemic brain injury, myocardial infarction, pancreatitis as well as liver and renal injury.8
Acute kidney injury (AKI) is still a critical problem in clinical practice, with a heavy health burden around the world.9 AKI affects ~13.3 million individuals and contributes to ~1.7 million deaths globally per year.10 Apoptosis was recognized as the main form of cell death that is responsible for renal dysfunction in AKI.11 Therefore, for a long time, strategies targeting the apoptosis pathway have been widely explored for AKI treatment. Despite the substantial therapeutic effect in animal models, the efficient anti-apoptosis intervention strategies are still absent in clinic. This could be partly ascribed to our limited understanding of the complex cell death mechanism in the process of AKI. Recent progress suggested that besides apoptosis, other forms of cell death, including regulated necrosis (such as, necroptosis, necrosis by mitochondrial permeability transition, pyroptosis, parthanatos and ferroptosis), autophagic cell death and mitotic catastrophe, also significantly contribute to the pathogenesis of AKI.12, 13 As the best-characterized form of regulated necrosis, necroptosis has been intensively explored in the setting of AKI. Understanding the precise role of necroptosis in AKI might provide potential therapeutic regimens. Therefore, this review summarized the potential role and corresponding mechanisms of necroptosis in AKI based on recent advances, and also discussed the possible therapeutic strategies based on manipulating necroptotic pathways.
The signaling pathways of necroptosis
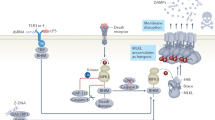
As the identification of necrostatins as specific inhibitors targeting receptor-interacting protein kinase 1 (RIP1), the signaling pathways of necroptosis have been extensively determined.14, 15 Engagement of death receptors and Toll-like receptors (TLRs), interferon (IFN) signals, as well as intracellular stimuli from protein DNA-dependent activator of IFN regulatory factors (DAI) in response to viruses are able to initiate necroptosis8 (Figure 1). Among them, tumor necrosis factor (TNF)-α-induced necroptosis in the presence of caspase inhibition is the best-characterized model. After TNF-α binds to TNF receptor (TNFR)1, the adaptor molecules Fas-associated death domain (FADD) and TNF-receptor-associated death domain recruit RIP1 that subsequently combines with RIP316, 17, 18 to form a complex termed ‘necrosome’.19 It is believed that the oligomerization driven by the RIP homotypic interaction motif (RHIM) domain on RIP3 and RIP1 leads to the autophosphorylation of RIP3, which results in the activation of RIP3.17, 18 In accordance, other initial participants like Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) and DAI use the RHIM domain to activate RIP3, indicating that RHIM domain has an important role in triggering necroptosis. Activated RIP3 recruits and phosphorylates the downstream MLKL, which serve as the executor of necroptosis.20, 21 It is demonstrated that phosphorylation of MLKL induces a molecular switch that enable MLKL to translocate to the membrane and consequently disrupt the plasma membrane. However, the exact mechanism needs to be further illustrated.7, 22 Moreover, it seems that MLKL could cause mitochondria fission via phosphoglycerate mutase family member 5 and dynamin-related protein 1.23 Whereas, the depletion of mitochondria has been determined not to prevent necroptosis in another study, suggesting that the mitochondrial fragmentation by MLKL may contribute little to the final execution of necroptosis.24
Schematic overview of necroptotic pathway. Necroptosis is triggered by various stimuli, including engagement of death receptors and Toll-like receptors, IFNs and intracellular protein DAI in response to viruses. Diverse upstream signals converge on mediator RIP3, and consequently activate the executor MLKL. Especially, an autocrine loop via de novo-transcribed IFNs/IFNAR1 signaling is believed to play a crucial role in sustaining the activation of necroptosis. Physiological regulators or pharmacological inhibitors can regulate necroptotic pathway at different molecular levels. Necrosulfonamide only acts on human MLKL
Other factors beyond TNF-α could also initiate RIP3-dependent necroptosis. It has been reported that activation of TLR3 or TLR4 by polyinosine–polycytidylic acid (poly(I:C)) and lipopolysaccharide (LPS), respectively, in the presence of zVAD (a pan-caspase inhibitor) leads to necroptosis through the adaptor protein TRIF, which contains RHIM domain to interact with RIP3.25, 26 Additionally, both type I (α/β) and type II (γ) IFNs were demonstrated to trigger necroptosis when FADD and caspase-8 are absent. IFNs-induced necroptosis proceeds by upregulating the expression of protein kinase R (PKR), which relies on JAK-STAT pathway. The PKR interacts with RIP1 and promotes the formation of the ‘PKR necrosome’ consisting of PKR, RIP1 and RIP3 to implement necroptosis.27 It should be noted that type I IFNs have been recently shown to have a predominant role in sustaining the activation of the RIP1–RIP3 complex.28 Macrophage deficient in IFN-α receptor (IFNAR) 1 was found to be resistant to LPS–TLR4-, poly(I:C)–TLR3- and TNF–TNFR1-mediated necroptosis in the presence of caspase inhibitors. Furthermore, it is proposed that LPS-, poly(I:C)-, TNF- and IFN-β-induced necroptosis in macrophages proceed through IFN regulatory factors-dependent de novo transcription of IFNs to facilitate the activation of necrosome. Also, the autocrine loops via de novo IFNs/IFNAR1 amplifies the initiating signals of necroptosis. Therefore, it is likely that type I IFNs provide a positive feedback to license the final execution of necroptosis. However, the ‘license’ role of type I IFNs in other cells need to be further validated.
Besides extrinsic pathways, intracellular signaling can also lead to necroptotic cell death. Viral infection-induced expression of cytosolic DAI could interact with RIP3 by RHIM domain and forms DAI–RIP3 complex with initiating function analogous to the RIP1–RIP3 necrosome or TRIF–RIP3 complex.29
These studies indicate that there exist diverse upstream pathways dependent on different stimuli. Various upstream signals converge on the RIP3 and share the same downstream executing pathway.
Key regulators of necroptosis
RIP1
Why Rip1-deficient mice die perinatally remains an intriguing question for a long time. Recent exciting findings showed that kinase-inactive Rip1 mutant D138N and K45A knockin mice are viable.30, 31 Importantly, in addition, cells derived from these mice are resistant to necroptosis, but mediate NF-κB pathway normally. These studies indicate that the kinase activity of RIP1 is indispensable and might serve as a ‘permission switch’ in the necroptotic pathways. Ironically, on the other hand, RIP1 itself might act as an inhibitor of necroptosis when its kinase activity is functionally absent. There probably exists an underlying physiological mechanism regulating the ‘permission switch’ of RIP1, thereby providing a negative feedback loop to restrict the magnitude of necroptosis. According to this theory, loss of RIP1 could result in the overreaction of necroptosis that might account for the lethality of Rip1-knockout mice. Consistently, Rip1−/− cells have high sensitivity to necroptotic stimuli.32 However, the proposed inhibitory effect of RIP1 needs further validation.
cIAPs, LUBAC and CYLD
In fact, not only necroptosis but also apoptosis and NF-κB pathways can be triggered by the engagement of TNFR1. During this process, cellular inhibitor of apoptosis proteins (cIAPs), linear ubiquitin chain assembly complex (LUBAC) and cylindromatosis (CYLD) are reported to have crucial roles in deciding the switch between different cellular outcomes.33, 34, 35, 36, 37 Briefly, TNFR1 signaling leads to the formation of distinct types of complexes with different functions. Polyubiquitination of RIP1 by cIAPs enables recruitment of LUBAC, which in turn stabilizes a so-called prosurvival complex (complex I) by generating the linear ubiquitin chains on RIP1. Subsequently, complex I leads to the well-known NF-κB signaling. Conversely, deubiquitination of RIP1 by CYLD or the absence of cIAPs and LUBAC renders complex I unstable and facilitates other complexes assembled to initiate apoptosis or necroptosis.
Complex of RIP1, FADD, caspase-8 and cFLIP isoforms
When cIAPs are absent, RIP1, FADD, caspase-8/10 and FADD-like interleukin (IL)-1β-converting enzyme (FLICE)-inhibitory protein (cFLIP) isoforms assemble an intracellular complex referred to as ripoptosome.38 Within the complex, cFLIP forms heterodimer with caspase-8, and controls the caspase activity. Depending on the isoforms of cFLIP, ripoptosome could lead to either apoptosis or necroptosis.38, 39, 40 cFLIPL (the long isoform of cFLIP)-caspase-8 heterodimer has restricted enzymatic activity that could inactivate RIP1 and RIP3 through cleavage, and consequently inhibits necroptosis and favors apoptosis; conversely, the heterodimer of caspase-8 and cFLIPS (the short isoform of cFLIP) lacks such catalytic activity and sensitizes cells to TLR- and Fas-induced necroptosis.38, 41 In the absence of cIAPs, RIP1 dissociates from complex I and forms an assembly consisting of RIP1, FADD, caspase-8 and long isoform of FADD-like interleukin (IL)-1β- converting enzyme (FLICE)-inhibitory protein (FLIPL), which favors apoptosis and inhibits spontaneous necroptosis. Consistently, Caspase-8-deficient mice die as embryos with heart defects,42 and this embryonic lethality can be rescued by Rip3 deletion.43 This phenomenon implies the negatively regulatory role of caspase-8 in necroptosis pathways. Of note, caspase-8 seems to inhibit necroptosis by forming a heterodimer with FLIPL.39, 40 High-expression level of FLIPL facilitates the formation of caspase-8–FLIPL complex, which cleaves and inactivates RIP1. Moreover, embryonic death of Fadd-deficient mice can also be prevented by Rip3 deletion, indicating that FADD also plays an inhibitory role.44, 45 Complex of RIP1, FADD, caspase-8 and FLIPL will divert to necrosome and necroptosis will be induced when caspase-8-FLIPL heterodimer is functionally defective or FADD is absent.
A20
RIP3 is required to be ubiquitinated at Lys5 to facilitate the formation of necrosome during necroptosis, while A20 could inhibit necroptosis by targeting this process.46 This theory is further validated by the phenomenon that loss of Rip3 reverses the lethality of A20-deficient mice.46
Necroptosis in AKI: evidence and implications
As shown in Figure 2, the presence of necroptosis in AKI was first determined in a murine model of renal ischemia–reperfusion injury (IRI) by Linkermann et al.47 who evaluated the protective effect of necrostatin-1 (Nec-1), a chemical inhibitor of RIP1. Nec-1 was shown to significantly alleviate the renal dysfunction and tissue damage, supporting the vital role of necroptosis in the pathogenesis of ischemic AKI. Surprisingly, compared with Nec-1, treatment with the pan-caspase inhibitor zVAD to block apoptosis did not provide any detectable protection. This finding contradicts with a previous report that demonstrated the protective effect of zVAD in the context of IRI.48 One possible reason is the incontinence in the clamping time of the renal pedicles adopted in these studies, which may result in diverse profiles of cellular death in kidneys. Besides, the different timing of zVAD administration may be another influence. It is known that apoptosis does not occur immediately after the onset of ischemia.11 Thus, it cannot be ruled out the possibility that application of zVAD just 15 min before ischemia might diminish its therapeutic effect. Both factors could be partially responsible for the ‘ineffectiveness’ of zVAD observed in this research. The role of necroptosis in ischemic kidney injury was further suggested in subsequent studies. Consistently, two researches confirmed the protection of Nec-1 in rat and human renal tubular epithelia cells (TECs), respectively, in a model of TNF-α stimulation and ATP depletion that mimics the renal ischemic injury in vitro.49, 50 Furthermore, genetic model provided more convincing evidence of necroptosis in renal IRI. Protection from ischemic damage was exhibited in Rip3-knockout mice, and further amelioration by addictive Nec-1 administration was not observed, implicating that Rip3-dependent necroptosis pathway participates in ischemic AKI as a crucial mediator.51 Rip3-deficient mice demonstrate a normal phenotype except for a slight failure to gain weight.52, 53 Notably, on the other hand, Rip3-knockout mice might also exert addictive protection due to its obviously increased peritubular diameters than wild-type mice.53 Therefore, tissue-specific Rip3-knockout models are needed to provide more explicit evidence. Taken together, these findings suggested that necroptosis is of crucial importance for the pathologic processes of renal IRI and targeting necroptotic pathway molecular emerges as a promising treatment option to improve the prognosis of ischemic AKI. However, the mechanism by which necroptosis contributes to renal IRI has not be fully delineated, which invites further investigations.
In addition to renal ischemic injury, necroptosis also contributes to AKI induced by cisplatin, a widely used chemotherapy agent with nephrotoxic effect. Tristao et al.54 found that zVAD prevents the cisplatin-associated damage on human renal TECs in vitro and combined use of zVAD and Nec-1 can provide additional protection. In agreement, Linkermann et al.51 showed an evident protection in Rip3-knockout mice in the background of cisplatin-induced AKI. Importantly, a more recent study by Xu et al.55 used Mlkl-knockout mice to investigate the role of necroptosis in AKI for the first time, providing more reliable evidence due to the indispensable role of MLKL in necroptotic pathway. Notably, it is shown that the increased production of TNF-α, TNF-related weak inducer of apoptosis (TWEAK) and IFN-γ (also known as TTI) in cisplatin-induced AKI may be an important promoter of necroptosis.55 However, whether TTI induces necroptosis of renal tissue in vivo directly in the background of cisplatin-associated AKI was not answered in this research. The nephrotoxicity of cisplatin remains a severe obstacle in its clinical application as an anticancer agent. One of the characteristics of cisplatin-induced AKI is the upregulation of proinflammatory cytokines.56 But to what extent these proinflammatory cytokines are related to necroptosis activation is unknown. More researches to address this question are urgently needed, which can optimize a combined therapeutic strategy to improve the efficacy of blocking necroptotic signaling.
Likewise, blocking necroptosis pathway by chemical inhibitor Nec-1 or gene knockout of key mediators can protect from AKI initiated by other nephrotoxic agents. For example, cyclosporin A (CsA) is a widely used immunosuppressive drug for organ transplantation and other autoimmune diseases. Necroptosis was also suggested in CsA-associated tubular injury with an in vitro model.57 The authors demonstrated obvious therapeutic effects of Nec-1 and knockdown of Rip3 in rat TECs exposed to CsA, indicating that necroptosis might also be implicated in the pathologic process of CsA-related AKI. Furthermore, Nec-1 was similarly shown to prevent from contrast-induced AKI in another study.58 Unfortunately, Nec-1 showed no relevance with cell death in this model but an unexpected effect on renal peritubular diameters. Thus, it cannot be excluded that the protection of Nec-1 is due to affecting renal blood flow but not blocking necroptosis pathways in this setting.
Recently, the contribution of necroptosis to AKI was determined in a glycerol-induced rhabdomyolysis model.59 The authors identified necroptosis, which is mainly mediated by increased circulating TNF, as the predominant form of tubular injury in this background. Meanwhile, cardiac injury was also observed in this model. Oppositely, necroptosis was detected to be of minor importance and apoptosis acts as a crucial mediator in the cardiac damage during rhabdomyolysis. This study exemplified the difference of organ-specific susceptibility to necroptotic cell death in the same circumstance. The overexpression of FLIP in kidneys may help drive the TNF signaling pathway towards necroptosis, but the exact mechanism remains to be illustrated.
Collectively, accumulating evidence has been extensively reported in recent years to demonstrate that necroptosis plays an crucial role in different types of AKI, suggesting a potential therapeutic target for this medical condition that needs to be clinically validated in the future (Tables 1 and 2).
Open questions
How to monitor necroptosis in vivo?
Substantial progress made in the past decade brought insights into the molecular mechanism of necroptosis, as well as its contribution to the pathogenesis of AKI. Until now, however, these studies have confined to preclinical models, and the diagnosis of necroptosis has still largely relied on detecting the protective effect of Nec-1 or knockout of Rip3 and Mlkl. However, protection of Nec-1 alone cannot provide a definite identification of necroptosis as its nonspecific functions on renal peritubular diameters, indolamin-2, 3-dioxygenase (IDO) and ferroptosis;58, 60, 61 and one, therefore, should be cautious when the presence of necroptosis is based only on the effect of Nec-1. Exploring the role of necroptosis in vivo requires reliable and feasible detection approaches. Antibodies that target phosphorylated MLKL through immunostaining or immunoblotting methods emerge as promising biomarkers to directly detect necroptosis. RIP3 could phosphorylate the activation loop of murine MLKL at Ser345, Ser347 and Thr349.62 A recent study from Rodriguez et al.63 indicated that phosphorylation of Ser345 is critical for RIP3-mediated necroptosis, during both the processes of MLKL activation and execution. They further generated a specific monoclonal antibody to detect phospho-Ser345 in murine cells.63 Distinctly, RIP3 phosphorylates human MLKL at the Thr357 and Ser358 sites.21 Wang et al.64 found that phosphorylation of both sites are essential for the engagement of necroptosis, and developed a monoclonal antibody that specifically recognizes human phospho-MLKL. This antibody has been applied to detect necroptosis in situ based on human liver biopsy samples from patients with drug-induced liver injury,64 although further validation in other disease models is required before its clinical application. To measure specific phosphorylation site of MLKL, other than to detect expression levels of pathway molecules, seems more efficient and accurate. It is inspired that commercial mAbs against murine and human phosphorylated MLKL are available now. Moreover, besides biopsy samples, biomarkers derived from blood or urine may also promise to offer clinically feasible tools to evaluate the dynamics of necroptosis in vivo. For instance, measurement of graft-derived circulating cell-free DNA has shown promise as a way to detect severe graft injury using droplet digital PCR.65 Hence, development of specific biomarkers is urgently needed in the future.
Necroptosis in AKI: when and where?
Some basic questions about necroptosis in AKI remain to be addressed. For example, whether and how necroptosis relates to different stages of AKI are still unknown. AKI consists of several mutually overlapping stages: initiation, extension, maintenance and recovery phases. Necroptosis may contribute to a variable degree to tissue injury and regeneration in various phases. Understanding this question enables better therapeutic strategies for AKI targeting at necroptosis. Recent knowledge recognizes necroptosis as a crucial participant in the early stage of AKI. In a previous study, furthermore, extra doses of the Nec-1 at 2 and 4 h after renal reperfusion followed ischemia that demonstrated no addictive protective effect.47 Despite of this observation, it cannot be concluded that necroptosis occurs in a snapshot fashion. The time course of necroptosis in various types of AKI is worthy of being further delineated.
Another question involves in the cell-specific sensitivity to necroptosis within kidney and its underlying mechanism. Compared with mesangial cells and podocytes, glomerular endothelial cells and tubular cell are more susceptible to TNF-triggered necroptosis in vitro.47 It is likely the expression level of RIP3 could serve as a predictor for cellular sensitivity towards necroptosis, whereas the exact regulating mechanism remains unclear. Understanding the precise susceptibility profiles of renal cells could help develop more efficient treatment aiming at certain cell types that are more vulnerable to necroptosis. More researches are required and tissue-specific gene deletion model should be used for better investigation of exact role of necroptosis in various renal cells during AKI.
How does necroptosis interplay with inflammation specifically during AKI?
Unlike apoptosis, necroptosis results in the release of unprocessed intracellular contents, also known as damage-associated molecular pattern (DAMP).22, 66 Subsequently, DAMP activates the innate immunity to produce more proinflammatory cytokines, which in turn leads to more necrosis in renal tissues. This autoamplification loop of inflammation is termed as necroinflammation;67, 68 and the conceptual advance very well delineates the process of immune cascades induced by necroptosis as well as other necrotic cell deaths. Specially, it is reported that necroptotic cells are characterized with increasing secretory IL-33.69 IL-33 is a pleiotropic cytokine of the IL-1 family and has an important role in tissue homeostasis.70 Increased level of extracellular IL-33 indicated that necroptosis might not be such proinflammatory as originally assumed. A recent study showed that liver-resident macrophages experience necroptosis during bacterial infection.71 Considering the abundance of tissue-resident immune cells within the kidney,72, 73, 74 whether they occur necroptosis upon various initiating stimuli remains an intriguing question. Furthermore, how does necroptosis orchestrate the dynamics of both resident and infiltrating immune cells? And how necroptosis is connected with the phased transition of immune cells from destructive to reparative role during AKI? All these questions need more studies in depth in the future.
What is the relative contribution of necroptosis and apoptosis in AKI?
Undoubtedly, necroptosis and apoptosis coexist in the pathophysiological process of AKI. However, whether necroptosis is relevant to the damage of kidney function during AKI are challenged recently. Despite some limitations in the detection techniques for apoptosis,11, 75 the effectiveness of anti-apoptosis therapeutic interventions in previous studies have proven the contribution of apoptosis to AKI,11 which should not be neglected even from current perspective. In fact, necroptosis, apoptosis and other modes of regulated cell death orchestrate the pathogenesis of AKI together; and the relative contribution of each cell death to AKI depends on the type and severity of the injury. The relative contribution of each one to the renal dysfunction in AKI remains elusive, and therefore exploring in more detail the contribution of necroptosis, apoptosis as well as other types of cell death in certain conditions of AKI will optimize specific therapeutic modalities.
Therapeutic implications
Identification of chemicals inhibiting the critical checkpoints makes it possible to manipulate necroptotic pathway. Nec-1, originally identified as the RIP1 inhibitor, has been widely used to treat necroptosis-associated diseases. However, a recent study has observed that Nec-1 could protect Rip1−/− cells from ferroptosis, indicating potential off-target effects of Nec-1 on ferroptosis.61 Besides, Nec-1 has yet unrecognized effects on renal peritubular diameters as mentioned above,58 and an unexpected inhibitory role on IDO.60 All these nonspecific functions of Nec-1 as well as the relatively short half-life period60 hamper its final clinical application. In spite of possible similar pharmacokinetic properties, a more-specific variant Nec-1 s that does not affect either ferroptosis or IDO provides a better alternative.60, 61 Beyond Nec-1(s), a series of necroptosis inhibitors have been reported recently.21, 26, 63, 76, 77, 78, 79, 80, 81 Particularly, two independent groups performed screens with a range of FDA-approved agents and identified three of them as potential drugs to block necroptosis. Dabrafenib was recognized as a selective inhibitor for RIP3,79 pazopanib for RIP1, and surprisingly, ponatinib for both RIP1 and RIP3.76 Given that the toxicities of pharmacological candidates are critical considerations in the clinical application, these drugs are more advantageous regarding this issue, for all of them are anticancer agents in current clinical use with well-documented side effects. However, whether such side effects are specifically acceptable for patients with necroptosis-associated diseases remains another question that warrants further elaborate evaluation. Considering the multiple initiating pathways at upstream levels, manipulating downstream mediators of necroptosis such as MLKL may be more effective. A direct inhibitor of human MLKL, necrosulfonamide, holds the promise to serve as a therapeutic drug.21 However, as many inhibitors interfering necroptosis have not been extensively explored to date, the efficacy and safety of these potential drugs should be further carefully validated.
In addition to inhibitors of key mediators, it is also possible to block necroptosis at the level of receptors. But the contribution of each receptor to differently induced AKI is uncertain, which complicates the development of a feasible and effective therapy. Thus the role of different receptors in various type of AKI must be clearly described in the future. Moreover, understanding the exact executive mechanism in the downstream of MLKL might lead to novel potential therapeutic checkpoints.
One should be cautious about the unwanted effect of necroptosis blockade on other forms of cell death or vice versa. For example, molecular pathways of necroptosis and apoptosis could crosstalk at various levels and therefore could mutually impact each other. This theory can be exemplified by zVAD that is shown to shift apoptosis to necroptosis.51 Therefore, more preclinical animal model researches are needed to clarify any possible reciprocal effects between necroptosis and other forms of cell death.
Theoretically, blocking necroptosis not only ameliorates cell death, but also reduces release of DAMPs as well as the subsequent inflammation, thus providing a promising therapeutic option. However, no necroptosis inhibitors are presently used for treatment of necroptosis-associated diseases in clinic. Although necroptosis inhibitors have entered clinical trials, several considerations should be seriously taken into account. Current data supported that an early intervention might exert a satisfying therapeutic effects, which is also implied by the necroinflammation theory. Unfortunately, such strategy might not be practical in a large fraction of cases, for AKI is usually asymptomatic at the early stage. This is why the time course of necroptosis discussed previously in the ‘open question’ section is particularly important. Because necroptosis can hardly be transient in AKI, the therapeutic window might be wide enough, which of course requires further validation. The establishment of such clinical trials in patients who have some certain predictable risk factors for AKI may be much easier, for example, kidney transplantation recipients. Some iatrogenic factors can also cause AKI, such as cardiac surgery. AKI has high incidence under these conditions, particularly among those with preexisting renal comorbidities (high-risk patients).82, 83 Therefore, prophylactic strategies are recommended under these situations. Meanwhile, the prophylactic strategy and early-onset intervention could be used in combination on a case-by-case basis, because the intensive management for these patients provides better detection for AKI in the early phase. It is reported that recipient mice receiving kidneys from Rip3-/- donors have longer survival and improved renal function.84 However, in clinic, it is not ethically or practically feasible to treat either living or deceased donors in kidney transplantation. Fortunately, cold ischemia time offers an ideal therapeutic window for preconditioning allografts with necroptosis inhibitors ex vivo. The trial designers should be precautious about the possible synergistic effects of necroptosis blockage and immunosuppression on acute severe infection.
A safety concern related to the increased risk of viral infection should be specially considered in the establishment of ‘anti-necroptosis’ clinical trials. The prevailing theory believes that necroptosis is a defensive mechanism against virus in physiological conditions.85 As a classic example, Rip3-deficient mice died during vaccinia virus infection.18 Although these are no sufficient evidence indicating that necroptosis is crucial in the control of whole spectrum of viruses and pharmacological inhibitors of necroptosis could result in viral infection, the safety concern regarding this problem remains a critical issue. Information on this potential risk should be included in the informed consent processes for participants in the clinical trials. In addition, careful screening for potential infections should be employed during anti-necroptosis treatment, especially for the recipients of renal transplantation.
Finally, given the fact that there are various and complex mechanisms contributing to AKI pathogenesis, it is reasonable to adopt a combination strategy. Blocking necroptosis in conjunction with current clinical treatment can be applied to improve the therapeutic efficacy and reduce side effects.
Conclusions
Taken together, necroptosis has been suggested to serve as a crucial role in AKI. The improving understanding of the underlying mechanism of necroptosis reveals a novel ‘checkpoint’ for AKI treatment. It is inspired that necroptosis inhibitors have entered clinical trials. And blocking necroptosis holds the great promise to improve the prophylaxis and prognosis of AKI in the clinical practice.
Abbreviations
- AKI:
-
acute kidney injury
- CsA:
-
cyclosporin A
- CYLD:
-
cylindromatosis
- DAI:
-
DNA-dependent activator of IFN regulatory factors
- DAMP:
-
damage-associated molecular pattern
- FADD:
-
Fas-associated death domain
- cFLIP:
-
cellular FADD-like interleukin (IL)-1β- converting enzyme (FLICE)-inhibitory protein
- cIAPs:
-
cellular inhibitor of apoptosis proteins
- IDO:
-
indolamin-2, 3-dioxygenase
- IFN:
-
interferon
- IFNAR:
-
IFN-α receptor
- IRI:
-
ischemia–reperfusion injury
- LPS:
-
lipopolysaccharide
- LUBAC:
-
linear ubiquitin chain assembly complex
- MLKL:
-
mixed lineage kinase domain-like protein
- Nec-1:
-
necrostatin-1
- poly(I:C):
-
polyinosine–polycytidylic acid
- PKR:
-
protein kinase R
- RHIM:
-
RIP homotypic interaction motif
- RIP1:
-
receptor-interacting protein kinase 1
- RIP3:
-
receptor-interacting protein kinase 3
- TLR:
-
toll-like receptor
- TNF:
-
tumor necrosis factor
- TNFR:
-
TNF receptor
- TWEAK:
-
TNF-related weak inducer of apoptosis
- TEC:
-
tubular epithelia cell
References
Clarke PG . Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990; 181: 195–213.
Danial NN, Korsmeyer SJ . Cell death: critical control points. Cell 2004; 116: 205–219.
Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ 2007; 14: 1237–1243.
Liu Y, Levine B . Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 2015; 22: 367–376.
Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P . Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 2008; 44: 205–221.
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P . Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15: 135–147.
Pasparakis M, Vandenabeele P . Necroptosis and its role in inflammation. Nature 2015; 517: 311–320.
Linkermann A, Green DR . Necroptosis. N Engl J Med 2014; 370: 455–465.
Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA et al. Acute kidney injury: an increasing global concern. Lancet 2013; 382: 170–179.
Mehta RL, Cerda J, Burdmann EA, Tonelli M, Garcia-Garcia G, Jha V et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015; 385: 2616–2643.
Havasi A, Borkan SC . Apoptosis and acute kidney injury. Kidney Int 2011; 80: 29–40.
Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z . Regulated cell death in AKI. J Am Soc Nephrol 2014; 25: 2689–2701.
Sancho-Martinez SM, Lopez-Novoa JM, Lopez-Hernandez FJ . Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin Kidney J 2015; 8: 548–559.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1: 112–119.
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008; 4: 313–321.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–336.
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137: 1100–1111.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–1123.
Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T . The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal 2010; 3: re4.
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 2012; 109: 5322–5327.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012; 148: 213–227.
Silke J, Rickard JA, Gerlic M . The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol 2015; 16: 689–697.
Wang Z, Jiang H, Chen S, Du F, Wang X . The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012; 148: 228–243.
Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 2013; 5: 878–885.
He S, Liang Y, Shao F, Wang X . Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA 2011; 108: 20054–20059.
Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 2013; 288: 31268–31279.
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci USA 2013; 110: E3109–E3118.
McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA 2014; 111: E3206–E3213.
Upton JW, Kaiser WJ, Mocarski ES . DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012; 11: 290–297.
Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014; 343: 1357–1360.
Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA 2014; 111: 7753–7758.
Kearney CJ, Cullen SP, Clancy D, Martin SJ . RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J 2014; 281: 4921–4934.
O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol 2011; 13: 1437–1442.
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA 2008; 105: 11778–11783.
Shimizu Y, Taraborrelli L, Walczak H . Linear ubiquitination in immunity. Immunol Rev 2015; 266: 190–207.
Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 2011; 471: 591–596.
Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell 2009; 36: 831–844.
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011; 43: 449–463.
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471: 363–367.
Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J 2011; 433: 447–457.
Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol 2009; 187: 1037–1054.
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9: 267–276.
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011; 471: 368–372.
Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J . Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 2011; 471: 373–376.
Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep 2012; 1: 401–407.
Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol 2015; 16: 618–627.
Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 2012; 81: 751–761.
Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 1999; 104: 541–549.
Zhang L, Jiang F, Chen Y, Luo J, Liu S, Zhang B et al. Necrostatin-1 attenuates ischemia injury induced cell death in rat tubular cell line NRK-52E through decreased Drp1 expression. Int J Mol Sci 2013; 14: 24742–24754.
Liang X, Chen Y, Zhang L, Jiang F, Wang W, Ye Z et al. Necroptosis, a novel form of caspase-independent cell death, contributes to renal epithelial cell damage in an ATP-depleted renal ischemia model. Mol Med Rep 2014; 10: 719–724.
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 2013; 110: 12024–12029.
Newton K, Sun X, Dixit VM. . Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 2004; 24: 1464–1469.
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 2014; 111: 16836–16841.
Tristao VR, Goncalves PF, Dalboni MA, Batista MC, Durao Mde S Jr., Monte JC . Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Ren Fail 2012; 34: 373–377.
Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 2015; 26: 2647–2658.
Ramesh G, Reeves WB . TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 2002; 110: 835–842.
Ouyang Z, Zhu S, Jin J, Li J, Qiu Y, Huang M et al. Necroptosis contributes to the cyclosporin A-induced cytotoxicity in NRK-52E cells. Pharmazie 2012; 67: 725–732.
Linkermann A, Heller JO, Prokai A, Weinberg JM, De Zen F, Himmerkus N et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol 2013; 24: 1545–1557.
Homsi E, Andreazzi DD, Faria JB, Janino P . TNF-alpha-mediated cardiorenal injury after rhabdomyolysis in rats. Am J Physiol Renal Physiol 2015; 308: F1259–F1267.
Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis 2012; 3: e437.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014; 16: 1180–1191.
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013; 39: 443–453.
Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ 2016; 23: 76–88.
Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 2014; 54: 133–146.
Oellerich M, Walson P, Beck J, Schmitz J, Kollmar O, Schutz E . Cell free DNA as a marker of transplant graft injury (liquid biopsy). Ther Drug Monit 2015 (e-pub ahead of print).
Kaczmarek A, Vandenabeele P, Krysko DV . Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 2013; 38: 209–223.
Linkermann A, Stockwell BR, Krautwald S, Anders HJ . Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 2014; 14: 759–767.
Mulay SR, Linkermann A, Anders HJ . Necroinflammation in kidney disease. J Am Soc Nephrol 2016; 27: 27–39.
Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014; 157: 1175–1188.
Molofsky AB, Savage AK, Locksley RM . Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015; 42: 1005–1019.
Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M . Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 2015; 42: 145–158.
Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D et al. Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-asialo-GM1 antibody. J Immunol 2015; 195: 4973–4985.
Park CO, Kupper TS . The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015; 21: 688–697.
Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J . Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol 2014; 10: 625–643.
Zeng W, Wang X, Xu P, Liu G, Eden HS, Chen X . Molecular imaging of apoptosis: from micro to macro. Theranostics 2015; 5: 559–582.
Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis 2015; 6: e1767.
Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA 2014; 111: 7391–7396.
Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 2014; 56: 481–495.
Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis 2014; 5: e1278.
Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA 2014; 111: 15072–15077.
Harris PA, Bandyopadhyay D, Berger SB, Campobasso N, Capriotti CA, Cox JA et al. Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med Chem Lett 2013; 4: 1238–1243.
Panek R, Tennankore KK, Kiberd BA . Incidence, etiology, and significance of acute kidney injury in the early post-kidney transplant period. Clin Transplant 2016; 30: 66–70.
Scrascia G, Rotunno C, Simone S, Montemurno E, Amorese L, De Palo M et al. Acute kidney injury in high-risk cardiac surgery patients: roles of inflammation and coagulation. J Cardiovasc Med (Hagerstown) 2015 (e-pub ahead of print).
Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A et al. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant 2013; 13: 2805–2818.
Kaiser WJ, Upton JW, Mocarski ES . Viral modulation of programmed necrosis. Curr Opin Virol 2013; 3: 296–306.
Acknowledgements
This study was supported by National Natural Science Foundation of China (grants 81400752 to CY, 81500572 to LH).
Author contributions
CY and LH conceived and designed the review. SW collected, analyzed literatures and wrote the draft. CZ helped to revise the language. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Edited by L Galluzzi
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, S., Zhang, C., Hu, L. et al. Necroptosis in acute kidney injury: a shedding light. Cell Death Dis 7, e2125 (2016). https://doi.org/10.1038/cddis.2016.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2016.37
This article is cited by
-
Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis
Laboratory Investigation (2018)
-
Wnt4 is significantly upregulated during the early phases of cisplatin-induced acute kidney injury
Scientific Reports (2018)
-
Identifying cisplatin-induced kidney damage in paediatric oncology patients
Pediatric Nephrology (2018)
-
Erythropoietin protects against rhabdomyolysis-induced acute kidney injury by modulating macrophage polarization
Cell Death & Disease (2017)
-
Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis
Cell Death & Disease (2017)