Abstract
Apoptosis is a major mode of cell death occurring during ischemia–reperfusion (I/R) induced injury. The p66Shc adaptor protein, which is mediated by PKCβ, has an essential role in apoptosis under oxidative stress. This study aimed to investigate the role of PKCβ2/p66Shc pathway in intestinal I/R injury. In vivo, ischemia was induced by superior mesenteric artery occlusion in mice. Ruboxistaurin (PKCβ inhibitor) or normal saline was administered before ischemia. Then blood and gut tissues were collected after reperfusion for various measurements. In vitro, Caco-2 cells were challenged with hypoxia–reoxygenation (H/R) to simulate intestinal I/R. Translocation and activation of PKCβ2 were markedly induced in the I/R intestine. Ruboxistaurin significantly attenuated gut damage and decreased the serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Pharmacological blockade of PKCβ2 suppressed p66Shc overexpression and phosphorylation in the I/R intestine. Gene knockdown of PKCβ2 via small interfering RNA (siRNA) inhibited H/R-induced p66Shc overexpression and phosphorylation in Caco-2 cells. Phorbol 12-myristate 13-acetate (PMA), which stimulates PKCs, induced p66Shc phosphorylation and this was inhibited by ruboxistaurin and PKCβ2 siRNA. Ruboxistaurin attenuated gut oxidative stress after I/R by suppressing the decreased expression of manganese superoxide dismutase (MnSOD), the exhaustion of the glutathione (GSH) system, and the overproduction of malondialdehyde (MDA). As a consequence, ruboxistaurin inhibited intestinal mucosa apoptosis after I/R. Therefore, PKCβ2 inhibition protects mice from gut I/R injury by suppressing the adaptor p66Shc-mediated oxidative stress and subsequent apoptosis. This may represent a novel therapeutic approach for the prevention of intestinal I/R injury.
Similar content being viewed by others
Main
Critical massive intestinal ischemia occurs in response to conditions such as acute mesenteric thrombotic or embolic occlusion, which are associated with high mortality.1, 2 Other gut ischemia cases followed by hemorrhagic shock, volvulus, sepsis, and abdominal aortic aneurysm surgery have a more subtle but no less damaging injury. Although restoration of the blood supply to the ischemic gut is critical to salvage, the reperfusion may paradoxically aggravate ischemic tissue damage and systemic inflammatory response.3 During the reperfusion period, a vicious cascade occurs including massive reactive oxygen species (ROS) generation, the activation of pro-apoptotic factors, and systemic inflammatory responses such as cytokine/chemokine release and polymorphonuclear neutrophil infiltration.4, 5 It becomes recognized that oxidative stress-induced ischemia/reperfusion (I/R) damage involves multiple signaling pathways.
PKC, a family of serine/threonine protein kinases comprising at least 12 members, has a central role in signal transduction and intracellular crosstalk.6 PKCβ1 and PKCβ2 isoforms are encoded by the same gene, PKCβ, and are not expressed in homozygous PKCβ−/− mice (NCBI Gene Database, identification number 18751). Multiple PKC isozymes are expressed in the intestine.7 Gene deletion or pharmacological blockade of PKCβ protects ischemic myocardium, decreases infarct size, and enhances recovery of ventricular function.8 Homozygous PKCβ-null mice and WT mice fed with ruboxistaurin (LY333531, selective PKCβ inhibitor) and subjected to single-lung I/R display increased survival, indicating that PKCβ has a pivotal role in the I/R-induced apoptosis.9 Despite these observations, the underlying mechanism by which PKCβ exerts deleterious effects in the intestinal I/R remains unclear.
The Shc adaptor protein family, consisting of the p66Shc, p52Shc, and p46Shc isoforms, is encoded by the ShcA locus.10 Due to the presence of a unique N-terminal domain (CH2), which is required for redox activity, p66Shc is the only isoform that acts as a redox enzyme implicated in mitochondrial ROS generation and the translation of oxidative signals into apoptosis.11 Phosphorylation at Ser36 of p66Shc is required for conferring increased susceptibility to oxidative stress, and is critical for the cell apoptosis elicited by oxidative damage.12 Migliaccio et al.11 reported the p66Shc−/− mouse increased resistance to oxidative stress and extended lifespan by 30%. Deletion of the p66Shc gene in mice is shown to protect hind limb,13 brain,14 and ex vivo hearts15 from I/R injury. It suggests that p66Shc would be a target to decrease the injury caused by intestinal I/R.
Hydrogen peroxide (H2O2) and hyperglycemic stress activate the PKCβ2 isoform to induce p66Shc phosphorylation at Ser-36, allowing transfer of the adaptor protein from the cytosol to the inner mitochondrial membrane, where it amplifies oxidative stress and catalyzes ROS production via cytochrome c oxidation.16, 17, 18 Therefore, we hypothesize that there may be a PKCβ2/p66Shc signaling pathway in the pathogenesis of intestinal I/R.
Results
Membrane translocation and phosphorylation of PKCβ2 in response to intestinal I/R
To test the hypothesis that PKC could be activated by I/R injury, we assessed cell membranous fraction of patterns for distinct PKC isoforms in the intestinal tissue subjected to 45 min ischemia followed by 45, 90, or 180 min reperfusion. A selective membrane translocation of PKCβ2 was detected, whereas PKCβ1, PKCδ, and PKCɛ showed no differences in membrane fraction after various reperfusion times (Figure 1a), indicating that PKCβ2 is specifically activated by I/R. To support this notion, we detected that a 90-min reperfusion significantly increased PKCβ2 phosphorylation at the thr-641 residue, leading to a markedly increased ratio of phosphorylated PKCβ2/total PKCβ2 (Figure 1b). These results demonstrated that both membrane translocation and activation of PKCβ2 occurred in the model of intestinal I/R.
Intestinal I/R-mediated membrane translocation and phosphorylation of PKCβ2. Mice were subjected to 45 min ischemia followed by 45, 90, or 180 min reperfusion. (a) Representative western blot demonstrating the expression of PKCβ1, PKCβ2, PKCδ, and PKCɛ in membranous fractions with Na,K-ATPase as a loading control. (b) Representative western blot demonstrating p-PKCβ2 (Thr 641) and total-PKCβ2 expression from sham and 90 min reperfusion intestine. All results are expressed as means±S.E.M., n=3 per group, **P<0.01 versus sham
Ruboxistaurin attenuates gut damage and the systemic inflammatory response after intestinal I/R
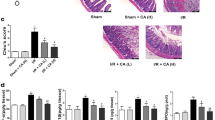
Next, ruboxistaurin (oral PKCβ inhibitor) and normal saline were given as a pretreatment before the superior mesenteric artery was occluded for 45 min followed by 90 min reperfusion. On examination of the histological changes, ruboxistaurin preserved the integrity of morphological structure well, and reduced both hemorrhage and neutrophil infiltration in the I/R intestine (Figure 2a). Similarly, the gut histological injury scores were significantly increased following I/R injury versus sham, and was reduced by ruboxistaurin (Figure 2b). Additionally, intestinal I/R significantly increased the serum levels of tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6). Ruboxistaurin, however, almost abrogated the increase in TNF-α and IL-6 concentrations (Figure 2c).
Ruboxistaurin pretreatment decreases the gut injury and the systemic inflammatory response after intestinal I/R. Normal saline and ruboxistaurin were given before sham and 45 min ischemia followed by 90 min reperfusion. (a) Gut tissues harvested after intestinal I/R were stained with hematoxylin and eosin, and examined under light microscopy at × 400 magnification. Representative images for sham, I/R, sham ruboxistaurin pretreatment, and I/R ruboxistaurin pretreatment groups. (b) Histologic injury scores of the gut in different groups were quantified as described in Materials and Methods. (c) Serum levels of TNF-α and IL-6 were determined by ELISA after intestinal I/R. All results are expressed as means±S.E.M., n=8 per group, **P<0.01 versus sham; ##P<0.01, #P<0.05 versus I/R. RBX, ruboxistaurin
Ruboxistaurin suppresses intestinal I/R-induced activation of PKCβ2 and p66Shc
Figure 3a showed that ruboxistaurin greatly suppressed the translocation of PKCβ2 in the I/R intestine over the same time period in which PKCβ1 was not impacted. Meanwhile, ruboxistaurin prevented the intestinal I/R-induced increase in the phosphorylation of PKCβ2 without affecting the expression of total PKCβ2, and suppressed the increased ratio of phosphorylated PKCβ2/total PKCβ2 (Figure 3b). Intestinal I/R moderately increased the expression of p66Shc, and greatly induced p66Shc phosphorylation. However, ruboxistaurin significantly reduced I/R-induced p66Shc overexpression and phosphorylation at ser36 (Figure 3c). Therefore, our study indicated that ruboxistaurin inhibited both PKCβ2 activation and PKCβ2-mediated p66Shc activation in the I/R intestine.
The effects of ruboxistaurin (oral PKCβ inhibitor) upon membrane distributions of PKCβ1 and PKCβ2, expression levels of p-PKCβ2 (Thr 641) and total-PKCβ2, and expression levels of p-p66Shc (Ser 36) and total-p66Shc in intestinal tissue. (a) Representative western blot demonstrating PKCβ1 and PKCβ2 expression in membrane fractions with Na,K-ATPase as a loading control. (b) Representative western blot demonstrating p-PKCβ2 and total-PKCβ2 expression. (c) Representative western blot demonstrating p-p66Shc and total-p66Shc expression with β-actin as a loading control. All results are expressed as means±S.E.M., n=3 per group, **P<0.01, *P<0.05 versus sham; ##P<0.01, #P<0.05 versus I/R. RBX, ruboxistaurin
Hypoxia/reoxygenation or phorbol 12-myristate 13-acetate-induced p66Shc activation: involvement of PKCβ2
Hypoxia/reoxygenation (H/R) of cells in vitro is a simple model of organ I/R, at least partly reflecting the pathophysiology in vivo. To simulate in vivo intestinal I/R, Caco-2 cells were exposed to H/R. To determine whether PKCβ2 is specifically required for the activation of p66Shc, we suppressed its expression using human-specific PKCβ2 small interfering RNA (siRNA) under normoxic and H/R conditions. Knockdown of PKCβ2 by siRNA reduced the expression of PKCβ2 and its phosphorylation in Caco-2 cells under normoxic and H/R conditions (Figure 4a). Our data showed that PKCβ2-siRNA had no effects upon p66Shc activation under normoxic conditions, but prevented p66Shc overexpression and phosphorylation under H/R conditions (Figure 4b). To further confirm whether p66Shc activation was activated by PKCβ2, we examined the effect of phorbol 12-myristate 13-acetate (PMA), a classical PKC activator, on the activation of p66Shc. The exposure of PMA markedly increased p66Shc phosphorylation in Caco-2 cells, which was inhibited significantly by PKCβ2 siRNA and ruboxistaurin (Figure 4c).
Expression of PKCβ2 and p66Shc in cultured Caco-2 cells following various treatments under normoxic or H/R conditions and PMA exposure. Caco-2 cells were incubated under hypoxic conditions for 15 h and then cultured under normoxic conditions for 6 h reoxygenation. Scrambled siRNA was used as a negative control. Representative western blot demonstrating (a) p-PKCβ2 and total-PKCβ2, and (b) p-p66Shc and total-p66Shc expression with β-actin as a loading control in Caco-2 cells transfected with PKCβ2 siRNA, exposed to normoxic or H/R conditions. (c) Representative western blot demonstrating p-p66Shc and total-p66Shc expression with β-actin as a loading control in Caco-2 cells transfected with PKCβ2 siRNA, exposed to PMA. All results are expressed as means±S.E.M., n=3 per group, **P<0.01, *P<0.05 versus sham; ##P<0.01 versus I/R; @P<0.05 versus all other groups
Inhibition of PKCβ2 activation by ruboxistaurin attenuates gut oxidative stress after intestinal I/R
To evaluate the oxidative state of the gut after I/R, we measured the levels of manganese superoxide dismutase (MnSOD), glutathione (GSH), glutathione peroxidase (GSH-PX), and malondialdehyde (MDA) in the intestinal tissues. Ruboxistaurin reversed intestinal I/R-induced anti-oxidant enzyme MnSOD downregulation (Figure 5a). ROS accumulation was increased in intestinal I/R tissue based on the assessment of MDA activity, which was reduced by ruboxistaurin (Figure 5b). In parallel, ruboxistaurin preserved intestinal I/R-induced GSH exhaustion and GSH-PX activity reduction (Figures 5c and d). Taken together, these data indicated that blockade of PKCβ2 decreased gut oxidative stress after intestinal I/R.
Inhibition of PKCβ2 activation by ruboxistaurin attenuates gut oxidative stress after intestinal I/R. (a) Representative western blot demonstrating MnSOD protein expression (n=3). (b) The activity of MDA in the intestine was determined. (c) The GSH levels in the intestine. (d) The GSH-PX levels in the intestine (n=8 per group for b, c, and d). All results are expressed as means±S.E.M., **P<0.01 versus sham; ##P<0.01, #P<0.05 versus I/R
Inhibition of PKCβ2 activation by ruboxistaurin inhibits gut apoptosis after intestinal I/R
To determine the apoptosis state of the gut after I/R, a terminal deoxynucleotidyl transferase mediated deoxyuridinetriphosphate nick end labeling (TUNEL) assay was conducted. The apoptotic cells in the gut were elevated from non-detectable to well observed after intestinal I/R, whereas ruboxistaurin significantly reduced the number of apoptotic cells (Figure 6a). In addition, ruboxistaurin significantly suppressed the increased levels of cleaved caspase-3, another marker of cell apoptosis, in the I/R intestinal tissue (Figure 6b).
Inhibition of PKCβ2 activation by ruboxistaurin inhibits gut apoptosis after intestinal I/R. (a) TUNEL staining of paraffin-embedded intestinal tissue sections. Representative images for sham, I/R, sham ruboxistaurin pretreatment, and I/R ruboxistaurin pretreatment groups (n=8). (b) Representative western blot demonstrating cleaved caspase-3 protein expression. All results are expressed as means±S.E.M., n=3 per group, **P<0.01 versus sham; ##P<0.01 versus I/R
Discussion
In the present study, we have demonstrated that I/R-induced intestinal dysfunction involved the PKCβ2/p66Shc signaling pathway. PKCβ2 activation played an essential role in the pathogenesis of intestinal I/R injury, and inhibition of excessive activation of PKCβ2 by ruboxistaurin reduced intestinal I/R injury at least partly via attenuation of the p66Shc activation. P66Shc acted as a redox enzyme implicated in mitochondrial ROS generation and the translation of oxidative signals into apoptosis. We provided evidence that pharmacological blockade or gene knockdown of PKCβ2 inhibited I/R-induced p66Shc activation, demonstrating that excessive p66Shc activation is associated with PKCβ2 activation. To the best of our knowledge, this is the first study examining the relationship between PKCβ2 and p66Shc in intestinal I/R.
Previous studies have reported activation of PKCβ2, PKCδ, and PKCɛ in cardiac ischemia or I/R,8, 19, 20 activation of PKCβ2 associated with the response to single-lung I/R,9 and activation of PKCδ and PKCɛ related to cerebral I/R.21 Our results demonstrated that the activated principal isoform of PKC in intestinal I/R was specifically PKCβ2, not PKCβ1, PKCδ, or PKCɛ (Figures 1a and b). These data suggested that the activation of individual PKC isoforms in ischemia or I/R is tissue specific. Moreover, our results indicated that in intestinal I/R, ruboxistaurin did not change the translocation of PKCβ1 over the same time period in which PKCβ2 was greatly impacted (Figure 3a). Taken together, it is likely that the primary role of ruboxistaurin was to inhibit the activation of PKCβ2 in intestinal I/R.
H/R significantly induces the activation of p66Shc, and ablation of p66Shc is cytoprotective against oxidative stress and apoptosis in hepatocytes.22 This may be clinically relevant as the mRNA level of p66Shc is increased in peripheral blood mononuclear cells of patients with acute myocardial infarction.23 In human aortic endothelial cells, selective inhibitor of PKCβ2 prevented p66Shc activation after exposed to hyperglycemic stress or oxidized low-density lipoprotein, respectively.18, 24 Our data showed that inhibition of PKCβ2 activation by ruboxistaurin attenuated p66Shc overexpression and phosphorylation at ser36 in the I/R intestine (Figure 3c). In vitro studies, knocking down PKCβ2 via siRNA inhibited the activation of PKCβ2, and further prevented p66Shc overexpression and phosphorylation under H/R conditions (Figure 4). By using both pharmacological blockade and gene knockdown PKCβ2 in vivo and in vitro experiments, we tested the above hypothesis that there may be a PKCβ2/p66Shc signaling pathway in intestinal I/R.
Gut I/R produces excessive amounts of ROS, which is responsible for the intestinal mucosa damage.25 Given exposure to ROS, mitochondrial proteins, lipids, and DNA are believed to be primary targets of oxidative damage, leading to alteration or loss of cellular functions, and causing inhibition of proliferation and induction of apoptosis.26 A growing body of evidence links p66Shc to oxidative stress as the adaptor protein has a pivotal role in modulating the intracellular redox state, increasing susceptibility to oxidative stress, and resulting in apoptosis elicited by oxidative damage.27, 28, 29 Our data demonstrated ruboxistaurin increased the intestinal I/R-induced downregulation of MnSOD, a primary ROS scavenging enzyme, but suppressed the accumulation of MDA, an indicator of lipid peroxidation (Figures 5a and b). Meanwhile, ruboxistaurin preserved intestinal I/R-induced GSH exhaustion and GSH-PX activity reduction (Figures 5c and d). Furthermore, the apoptosis execution enzyme caspase-3 has a crucial role in cell apoptosis by resulting in DNA fragmentation, degradation of cytoskeleton, and formation of apoptotic bodies. Arany et al.30 showed that p66Shc was associated with cytochrome c, which is responsible for the activation of caspase-3 in the kidneys of mice with I/R injury. Our data showed that ruboxistaurin significantly attenuated intestinal caspase-3 activity and inhibited the apoptosis of the intestine subjected to I/R (Figures 6a and b). Therefore, it is conceivable that the inhibition of PKCβ2 activation by ruboxistaurin attenuates p66Shc-mediated oxidative stress and subsequent apoptosis in intestinal I/R.
During the reperfusion period, mucosal barrier integrity is destroyed and the systemic release of pro-inflammatory cytokines occurs, with concurrent leukocyte activation and bacterial translocation.31 In this study, intestinal I/R injury significantly increased the serum levels of TNF-α and IL-6, suggesting that a severe systemic inflammation response was induced during the reperfusion period. Ruboxistaurin administration almost abrogated the increase in TNF-α and IL-6 serum concentration (Figure 2c).
Ruboxistaurin, an oral PKCβ inhibitor, is currently undergoing phase 2 and phase 3 clinical testing for several cardiovascular diseases, such as diabetic retinopathy and diabetic kidney disease.32, 33 Due to be administrated orally, ruboxistaurin was gavaged for 3 days before I/R, which would be a potential limitation in acute clinical cases. However, the focus of this study was to investigate the role of PKCβ2 in regulating p66Shc-mediated intestinal I/R injury.
In summary, our results demonstrate that the inhibition of PKCβ2 activation attenuated intestinal I/R injury and systemic inflammation response by inhibiting the adaptor p66Shc-mediated oxidative stress and subsequent apoptosis. Furthermore, the activated principal isoform of PKC in intestinal I/R was specifically PKCβ2, not PKCβ1, PKCδ, or PKCɛ. These may represent a novel therapeutic avenue for intestinal I/R injury.
Materials and Methods
Murine model of intestinal I/R
Male ICR mice (aged 4 weeks) weighing 20±2 g were obtained from the Animal Center of Dalian Medical University (Dalian, China), and kept under standard laboratory conditions with standard laboratory chow and water. The mouse intestinal occlusion-and-reperfusion procedure was performed as described previously.5 Briefly, the superior mesenteric artery was occluded by a microvascular clamp for 45 min and then 45, 90, or 180 min reperfusion was performed. Normal saline and ruboxistaurin (LY 333531; ENZO, Lausen, Switzerland) were given by oral gavage before sham and 45 min ischemia, followed by 90 min reperfusion at a dose of 10 mg/kg daily for 3 days (demonstrated to adequately inhibit PKCβ activation in mice heart and vasculature).9 All procedures were conducted according to the Institutional Animal Care Guidelines, and were approved by the Institutional Ethics Committee.
Histological and TUNEL staining
For histological and TUNEL analysis, formalin-fixed tissues were embedded in paraffin and sectioned. The 4-μm sections were stained by hematoxylin–eosin. Intestinal I/R-induced mucosal injury was evaluated according to Chiu’s score.34 TUNEL staining was performed using an apoptosis assay kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions.
Measurement of cytokines
The levels of serum TNF-α and IL-6 were measured using Enzyme-linked immunosorbent assay (ELISA) kits (ENGTON Bio-engineering Limited Company, Shanghai, China), according to the manufacturer’s protocols.
Intestinal GSH, GSH-PX, and MDA activity assay
The GSH and GSH-PX activities were determined using an assay kit (Nanjing Jiancheng Corp., Nanjing, China), according to the manufacturer’s recommendations. The level of MDA in the intestinal tissues was quantified by a lipid peroxidation MDA assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer’s protocol.
Cell culture
Caco-2 cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 in DMEM, supplemented with 10% fetal bovine serum, 1% non-essential amino acids, and 1% glutamide (Gibco, Carlsbad, CA, USA). To simulate physiologic conditions, Caco-2 cells were grown as monolayers on platforms, providing both apical and basolateral areas, thereby allowing cells to become polarized. The culture medium was then replaced with serum-free DMEM before experimental treatment.
Transient transfection of siRNA
Caco-2 cells (1 × 105) were seeded on six-well plates and transfected at the time of 70–80% confluence with a PKCβ2 siRNA or non-binding control siRNA using Lipofectamin 2000 (Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. The siRNA which was used to target PKCβ2 had the sequences: 5′-GCGACCUCAUGUAUCACAUdTdT-3′ and 5′-AUGUGAUACAUGAGGUCGCdTdT-3′ (Genepharma, Shanghai, China). Scrambled siRNA which was used as a negative control had the sequences: 5′-ACGUGACACGUUCGGAGAAdTdT-3′ and 5′-UUCUCCGAACGUGUCACGUdTdT-3′. Commercial PKCβ2 siRNA was utilized for the inhibition of PKCβ2 expression as per manufacturer’s protocol.
H/R incubation and PMA exposure
To simulate in vivo intestinal ischemia, unless otherwise noted, cellular hypoxic conditions were created. For the hypoxic conditions, cells were incubated in a microaerophilic system (Thermo Fisher Scientific 8000, Marietta, GA, USA) at 5% CO2 and 1% O2, and balanced with 94% N2 gas for 15 h.35 The cells were then cultured in normoxic conditions for 6 h of reoxygenation. After transfection with control or PKCβ2 siRNA, cells were incubated in either normoxic or H/R DMEM medium. Caco-2 cells were exposed to 100 nM PMA (Sigma-Aldrich, St. Louis, MO, USA) for 30 min in the absence or in the presence of PKCβ2 siRNA or ruboxistaurin (20 nM).
Western blot analysis
Equal protein amounts from isolated intestinal tissue and Caco-2 cell homogenate were removed using 10–15% SDS-PAGE (Bio-Rad, Hercules, CA, USA), and subsequently transferred onto PVDF membrane (Millipore, Bedford, MA, USA). Antibodies used for western blotting included those for PKCβ1, PKCβ2, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); PKCδ, PKCɛ, cleaved caspase-3, and Na,K-ATPase (Bioworld Technology, St. Louis Park, MN, USA); phospho-PKCβ2 (Cell Signaling Technology, Danvers, MA, USA); and p66Shc, phospho-p66Shc, and MnSOD (Abcam Ltd., Cambridge, UK). Appropriate secondary antibodies were used to detect the primary antibody/antigen complexes. The membranes were exposed to enhanced chemiluminescence-plus reagents (Beyotime Institute of Biotechnology). Emitted light was documented using a multispectral imaging system (UVP, Upland, CA, USA), and gels were analyzed using a Gel-Pro Analyzer, Version 4.0 (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Densitometry was obtained by the image analysis software (UVP). All values are presented as means±S.E.M. The data were analyzed with a two-tailed Student’s t-test when comparing means between two groups. One-way analysis of variance (ANOVA) followed by Student–Newman–Keuls (SNK) test was used when comparing multiple groups. The ordinal values of the gut injury scores were analyzed by the Kruskal–Wallis non-parametric test. Statistical analysis was performed by the GraphPad Prism (version 5.0; GraphPad Prism Software, La Jolla, CA, USA). P-values less than 0.05 were considered as significant.
Abbreviations
- I/R:
-
ischemia/reperfusion
- H/R:
-
hypoxia/reoxygenation
- TNF-α:
-
tumor necrosis factor-α
- IL-6:
-
interleukin 6
- siRNA:
-
small interfering RNA
- PMA:
-
Phorbol 12-myristate 13-acetate
- MnSOD:
-
manganese superoxide dismutase
- MDA:
-
malondialdehyde
- GSH:
-
glutathione
- GSH-PX:
-
glutathione peroxidase
- ROS:
-
reactive oxygen species
References
Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M . Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg 2005; 241: 516–522.
Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD . Acute mesenteric ischemia: a clinical review. Arch Intern Med 2004; 164: 1054–1062.
Gibot S, Massin F, Alauzet C, Montemont C, Lozniewski A, Bollaert PE et al. Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit Care Med 2008; 36: 504–510.
Wu B, Qiu W, Wang P, Yu H, Cheng T, Zambetti GP et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut 2007; 56: 645–654.
Chen Y, Lui VC, Rooijen NV, Tam PK . Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut. Gut 2004; 53: 1772–1780.
Mellor H, Parker PJ . The extended protein kinase C superfamily. Biochem J 1998; 332: 281–292.
Tuo BG, Chow JY, Barrett KE, Isenberg JI . Protein kinase C potentiates cAMP-stimulated mouse duodenal mucosal bicarbonate secretion in vitro. Am J Physiol Gastrointest Liver Physiol 2004; 286: G814–G821.
Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ et al. PKCbeta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol 2008; 294: H1862–H1870.
Fujita T, Asai T, Andrassy M, Stern DM, Pinsky DJ, Zou YS et al. PKCbeta regulates ischemia/reperfusion injury in the lung. J Clin Invest 2004; 113: 1615–1623.
Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G et al. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J 1997; 16: 706–716.
Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999; 402: 309–313.
Pacini S, Pellegrini M, Migliaccio E, Patrussi L, Ulivieri C, Ventura A et al. p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Mol Cell Biol 2004; 24: 1747–1757.
Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation 2004; 109: 2917–2923.
Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J 2013; 34: 96–103.
Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M . The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta 2009; 1787: 774–780.
Nemoto S, Finkel T . Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002; 295: 2450–2452.
Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 2007; 315: 659–663.
Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M et al. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res 2012; 111: 278–289.
Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 2003; 108: 2304–2307.
Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T et al. Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation 2004; 110: I194–I199.
Bright R, Mochly-Rosen D . The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 2005; 36: 2781–2790.
Haga S, Terui K, Fukai M, Oikawa Y, Irani K, Furukawa H et al. Preventing hypoxia/reoxygenation damage to hepatocytes by p66(shc) ablation: up-regulation of anti-oxidant and anti-apoptotic proteins. J Hepatol 2008; 48: 422–432.
Franzeck FC, Hof D, Spescha RD, Hasun M, Akhmedov A, Steffel J et al. Expression of the aging gene p66Shc is increased in peripheral blood monocytes of patients with acute coronary syndrome but not with stable coronary artery disease. Atherosclerosis 2012; 220: 282–286.
Shi Y, Cosentino F, Camici GG, Akhmedov A, Vanhoutte PM, Tanner FC et al. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase C-beta, and c-Jun N-terminal kinase kinase in human endothelial cells. Arterioscler Thromb Vasc Biol 2011; 31: 2090–2097.
Sukhotnik I, Brod V, Lurie M, Rahat MA, Shnizer S, Lahat N et al. The effect of 100% oxygen on intestinal preservation and recovery following ischemia-reperfusion injury in rats. Crit Care Med 2009; 37: 1054–1061.
Finkel T, Holbrook NJ . Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408: 239–247.
Gertz M, Steegborn C . The Lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxid Redox Signal 2010; 13: 1417–1428.
Beltrami E, Ruggiero A, Busuttil R, Migliaccio E, Pelicci PG, Vijg J et al. Deletion of p66Shc in mice increases the frequency of size-change mutations in the lacZ transgene. Aging Cell 2013; 12: 177–183.
Xiong Y, Yu Y, Montani JP, Yang Z, Ming XF . Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: implications for atherosclerotic plaque vulnerability. J Am Heart Assoc 2013; 2: e96.
Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y . p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol 2010; 298: F1214–F1221.
Grootjans J, Hundscheid IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK et al. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 2013; 62: 250–258.
Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD et al. Oral protein kinase c beta inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study 2. Retina 2011; 31: 2084–2094.
Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW . Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2007; 2: 631–636.
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN . Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970; 101: 478–483.
Baylor AR, Diebel LN, Liberati DM, Dulchavsky SA, Brown WJ, Diglio CA . The synergistic effects of hypoxia/reoxygenation or tissue acidosis and bacteria on intestinal epithelial cell apoptosis. J Trauma 2003; 55: 241–247 247-248.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81171850 and 81372037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Federici
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Chen, Z., Wang, G., Zhai, X. et al. Selective inhibition of protein kinase C β2 attenuates the adaptor P66Shc-mediated intestinal ischemia–reperfusion injury. Cell Death Dis 5, e1164 (2014). https://doi.org/10.1038/cddis.2014.131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2014.131
Keywords
This article is cited by
-
PKCζ phosphorylates TRAF2 to protect against intestinal ischemia–reperfusion–induced injury
Cell Death & Disease (2017)
-
MicroRNA-378 protects against intestinal ischemia/reperfusion injury via a mechanism involving the inhibition of intestinal mucosal cell apoptosis
Cell Death & Disease (2017)
-
MicroRNA-682-mediated downregulation of PTEN in intestinal epithelial cells ameliorates intestinal ischemia–reperfusion injury
Cell Death & Disease (2016)
-
Blockade of PKCβ protects against remote organ injury induced by intestinal ischemia and reperfusion via a p66shc-mediated mitochondrial apoptotic pathway
Apoptosis (2014)