Abstract
Obesity is associated with chronic low-grade inflammation and oxidative stress that blunt insulin response in its target tissues, leading to insulin resistance (IR). IR is a characteristic feature of type 2 diabetes. Skeletal muscle is responsible for 75% of total insulin-dependent glucose uptake; consequently, skeletal muscle IR is considered to be the primary defect of systemic IR development. Interestingly, some obese people stay insulin-sensitive and metabolically healthy. With the aim of understanding this difference and identifying the mechanisms responsible for insulin sensitivity maintenance/IR development during obesity, we explored the role of the latent endoribonuclease (RNase L) in skeletal muscle cells. RNase L is a regulator of innate immunity, of double-stranded RNA sensors and of toll-like receptor (TLR) 4 signaling. It is regulated during inflammation by interferons and its activity is dependent on its binding to 2-5A, an oligoadenylate synthesized by oligoadenylate synthetases (OAS). Increased expression of RNase L or downregulation of its inhibitor (RLI) improved insulin response in mouse myogenic C2C12 cells and in primary human myotubes from normal-weight subjects treated with palmitate, a saturated free fatty acid (FFA) known to induce inflammation and oxidative stress via TLR4 activation. While RNase L and RLI levels remained unchanged, OAS level was decreased in primary myotubes from insulin-resistant obese subjects (OB-IR) compared with myotubes from insulin-sensitive obese subjects (OB-IS). TLR3 and mitochondrial manganese superoxide dismutase (MnSOD) were also underexpressed in OB-IR myotubes. Activation of RNase L by 2-5A transfection allowed to restore insulin response, OAS, MnSOD and TLR3 expression in OB-IR myotubes. Due to low expression of OAS, OB-IR myotubes present a defect in RNase L activation and TLR3 regulation. Consequently, MnSOD level is low and insulin sensitivity is reduced. These results support that RNase L activity limits FFA/obesity-induced impairment of insulin response in muscle cells via TLR3 and MnSOD expression.
Similar content being viewed by others
Main
Obesity or high-fat diet conditions are associated with an increased risk to develop metabolic dysfunctions such as insulin resistance (IR), which is a central determinant of type 2 diabetes (T2D) pathogenesis.1 Nevertheless, in about 30% of obese individuals, insulin sensitivity is preserved,2, 3 the mechanisms allowing these obese people to stay metabolically healthy being not yet clearly understood.
Since the 50s, several studies have led to develop the idea that IR is a major consequence of the establishment of a chronic low-grade inflammatory state and oxidative stress.4, 5 Excess of saturated free fatty acids (FFA) overly activates toll-like receptors (TLR), that leads to the local activation of innate immune response.6, 7 It results in production and release of numerous cytokines such as type I interferons (IFNI), interleukin (IL)-1β, IL-6 and tumor necrosis factor α (TNFα), and chemokines such as monocyte chemoattractant protein-1 (MCP-1), which activate inflammation and oxidative stress. However, the specific mechanisms linking TLR activation, inflammation, oxidative stress and defect in insulin sensitivity have been only partially characterized.
Inflammation induces the expression and the activation of inflammatory kinases, such as c-Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPK) and double-stranded RNA-dependent protein kinase (PKR), which both phosphorylate insulin receptor substrate 1 (IRS1) on serine residues, thus disrupting insulin signaling.8, 9 Moreover, these kinases activate another one, the inhibitor of κB (IκB) kinase (IKK), or directly phosphorylate IκB, leading to activation of transcriptional nuclear factor κB (NF-κB) and expression of inflammatory genes.10
In parallel, chronic FFA exposure is associated with increased reactive oxygen species (ROS) production (the main source being the mitochondria), through a TLR4-dependent mechanism that also involves innate immunity response and the release of pro-inflammatory cytokines.11, 12, 13 Besides, ROS and in particular hydrogen peroxide (H2O2) are essential for many biological processes including regulation of insulin signal transduction.4, 14 Indeed, small amount of H2O2 is produced during insulin receptor stimulation and facilitates normal insulin signal transduction to the different proteins of the signaling cascade via tyrosine phosphorylation (e.g., insulin receptor, IRS1) or serine phosphorylation (protein kinase B (PKB)/Akt).15, 16 If ROS are indispensable for the proper insulin response, their excess may however cause its disruption and contribute to IR and T2D, in particular because they lead to JNK activation.17, 18 Therefore, ROS level must be tightly regulated. Pro-oxidants are naturally neutralized or detoxified by enzymes such as superoxide dismutases (SOD), glutathione peroxidases (GPx) and catalase, ‘oxidative stress’ resulting from an imbalance between ROS generation and anti-oxidant defenses.
Innate immunity, inflammation and oxidative stress processes are finely controlled by the induction of negative regulators to avoid unchecked immune reactions. IFNI have an important role in the balance between activation and inhibition of immune responses, particularly through their anti-inflammatory properties such as inhibition of TNFα and regulation of IL-10 production.19 The mechanism of this positive effect of IFNI on insulin signaling has not yet been studied in depth.
The latent endoribonuclease (RNase L) is an essential actor of innate immunity and its activity is regulated by IFNI.20, 21 During inflammation, the induction of its activity is essential for TLR4 signal transduction22 and for activation of double-stranded RNA sensors such as retinoic acid-inducible gene-1 (RIG-1) and melanoma differentiation-associated gene 5 (MAD5).21 RNase L activity is strictly dependent on its binding by small oligoadenylates, the 2-5A, which are synthesized by the 2-5A oligoadenylate synthetases (OAS), OAS1 and OAS2. OAS expression is also induced by IFNI during inflammation.20 RNase L, when activated by 2-5A, cleaves single-stranded RNA at UpNp sequences during their translation, inhibiting protein synthesis.23 Via their secondary structures, these cleaved mRNA could also themselves regulate another RNA sensor pathway initiated by TLR3 activation.21, 24 TLR3 activation leads to IFNI production and expression of IFN-stimulated genes (ISG) such as OAS genes, thus to 2-5A synthesis.25 The 2-5A activate RNase L, leading to the amplification of TLR3 signaling. Moreover, IFNI, in turn, induce TLR3 expression in a positive amplification loop.26 RNase L activity is negatively regulated by RNase L inhibitor/ATP-binding cassette sub-family E member 1 (RLI/ABCE1).27 We previously demonstrated the major role of RNase L and RLI in lipids accumulation, cell fate and insulin response during myogenesis and adipogenesis via regulation of MyoD and C/EBP (CCAAT-enhancer-binding protein)-homologous protein 10 (CHOP10) mRNA expression.28, 29, 30
Skeletal muscle is an essential organ for insulin action and glucose metabolism, and is the primary site of peripheral IR development.31 It is well documented that elevation of plasma FFA is currently associated with obesity and causes inflammation, oxidative stress and IR in skeletal muscle.32, 33 The current study was performed to investigate the implication of RNase L/RLI in FFA-induced IR in skeletal muscle cells during obesity. Our results show that RNase L has an important role in maintaining insulin response during inflammation in mouse and human muscle cells. Myotubes from obese insulin-resistant (OB-IR) individuals, when compared with those from obese insulin-sensitive (OB-IS) individuals, are characterized by a lower expression of the enzymes manganese SOD (MnSOD) and OAS. Cell transfection with 2-5A allows to restore insulin response as well as MnSOD expression in OB-IR myotubes. Moreover, investigating the mechanism by which RNase L regulates insulin response led us to demonstrate that reduced expression of MnSOD and OAS is linked to low level of TLR3. We identify here a mechanism regulated by inflammation which allows maintenance of insulin response via TLR3 regulation and accounts for the difference between insulin-sensitive and insulin-resistant obese people’s muscle.
Results
RNase L limits deleterious effect of palmitate on insulin signaling in mouse myogenic cells
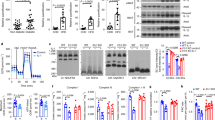
To determine the impact of RNase L/RLI expression on insulin signaling during inflammation, we evaluated insulin response in C2C12-derived mouse myogenic cells expressing different levels of RNase L or RLI, after FFA treatment. The saturated FFA palmitate was used here to mimic the effect of elevated circulating FFA, known to induce an acute pro-inflammatory response via TLR pathway in obesity.34 As previously described28, 30 and shown in Figure 1a, RNase L and RLI expression was significantly increased in C2-RNaseL+ (*P=0.028) and C2-RLI (*P=0.024), respectively, compared with C2-RNaseL control cells.
Insulin response in C2C12-derived mouse myogenic cells after palmitate treatment. (a) RNase L expression was IPTG-induced (C2-RNaseL+) or not in differentiated C2-RNaseL cells. Total proteins were extracted from C2-RNaseL, C2-RNaseL+ and C2-RLI, and RNase L and RLI expression was assessed by western blotting. A membrane scan obtained in each cell line is presented in the figure. RNase L and RLI levels in control C2-RNaseL were set at 100%. Error bars refer to the S.E.M. obtained in three independent experiments. *P≤0.05. (b) Differentiated C2-RNaseL, C2-RNaseL+ and C2-RLI cells were incubated or not with palmitate then stimulated or not with insulin. To measure insulin response, P-Akt and Akt proteins expression was assessed on total cellular extracts by western blotting. A membrane scan obtained in each cell line is presented in the figure. The control value, with neither palmitate treatment nor insulin stimulation, was set at 100% for each cell line. Error bars refer to the S.E.M. obtained in three independent experiments. **P<0.01
Without induction of inflammation, insulin response was totally abolished in cells overexpressing RLI (C2-RLI), as shown by no increase in P-Akt/Akt expression rate following insulin stimulation, while Akt phosphorylation was induced in the two other C2C12-derived cell lines (Figure 1b). In basal conditions, we observed no any difference in insulin response between cells overexpressing RNase L (C2-RNaseL+) and C2-RNaseL. This could be explained on account of the fact that, without inflammation induction, insulin response was already high in C2-RNaseL. On the other hand, palmitate treatment blunted insulin response in C2-RNaseL and C2-RNaseL+, C2-RNaseL+ however maintaining a significantly higher level of P-Akt/Akt expression rate than C2-RNaseL (Figure 1b). As expected, there was no insulin-stimulated Akt phosphorylation in C2-RLI after palmitate exposure (Figure 1b).
Downregulation of RLI expression impacts palmitate-induced impairment of insulin response in myotubes from normal-weight subjects
Human primary myotubes obtained from skeletal muscle of normal-weight subjects were transfected with specific RNase L (Myo-siRNaseL) or RLI (Myo-siRLI) siRNA or with a non-specific siRNA (Myo-siControl) before palmitate treatment and/or insulin stimulation.
In Myo-siControl, palmitate treatment induced RNase L and RLI proteins expression without increasing RNase L or RLI mRNA levels (Figures 2a and b). This indicates that the induction of these proteins would be the result of a post-transcriptional regulatory mechanism such as increased protein translation, as yet demonstrated for RNase L.35
Insulin response of normal-weight subjects’ myotubes after siRNA transfection and palmitate treatment. (a) Human myotubes were transfected with control siRNA (Myo-siControl) or specific siRNA against RLI (Myo-siRLI) or RNase L (Myo-siRNaseL) and were then incubated or not with palmitate. Total RNA were then extracted from siRNA-transfected cells to measure expression of RNase L and RLI mRNA by q-PCR. For each mRNA, the mean level of mRNA expression in Myo-siControl was set at 1. Error bars refer to the S.E.M. obtained from five samples issued from different donors. *P<0.05 and **P<0.01. (b) Human myotubes were siRNA-transfected and were then incubated or not with palmitate. RNase L and RLI proteins expression was assessed on total cellular extracts by western blotting. Membrane scans obtained from two different samples are presented in the figure. RNase L and RLI levels in Myo-siControl without palmitate treatment were set at 100%. Error bars refer to the S.E.M. obtained from five samples issued from different donors. *P≤0.05. (c) siRNA-transfected human myotubes were incubated or not with palmitate then stimulated or not with insulin. P-Akt and Akt proteins expression was assessed on total cellular extracts by western blotting. Membrane scans obtained from two different samples of each siRNA-transfected cell population are presented in the figure. Data are expressed relative to the fold induction of P-Akt/Akt protein level in each cell population with neither palmitate treatment nor insulin stimulation (basal response), which was set at 1. Error bars refer to the S.E.M. obtained from five samples issued from different donors. *P<0.05
Both RNase L and RLI mRNA levels were significantly lower in Myo-siRNaseL (*P=0.039) and Myo-siRLI (*P=0.029), respectively, compared with Myo-siControl, demonstrating the effectiveness of siRNA transfection, that is, specific mRNA degradation (Figure 2a). However, these significant differences were not confirmed at protein level, suggesting a low RNase L and RLI proteins turnover and/or a stable expression of these proteins, as it has already been observed by others for RNase L.36 On the other way, induction of RNase L and RLI proteins expression following palmitate treatment was not observed in Myo-siRNase L and Myo-siRLI, respectively. Indeed, in palmitate-treated cells, RNase L and RLI expression was significantly lower in Myo-siRNaseL (*P=0.014) and Myo-siRLI (*P=0.05), respectively, compared with Myo-siControl (Figure 2b). These results, which show an inhibition by siRNA of palmitate-induced RNase L and RLI proteins translation, would be explained by the fact that siRNA can regulate gene expression through both mRNA stability and mRNA translation.37
Palmitate treatment similarly impaired insulin signaling in Myo-siControl and Myo-siRNaseL, as shown by a relative fold expression of insulin-stimulated P-Akt/Akt rate inferior to 1 (0.75±0.07 and 0.81±0.10, respectively), while it was significantly maintained and even increased in Myo-siRLI (1.41±0.30) (Figure 2c; *P=0.032). The absence of RNase L siRNA effect on insulin response in Myo-siRNaseL could be explained by the fact that, at these concentrations of palmitate, inhibition of insulin response is yet maximal (compare insulin-stimulated P-Akt/Akt expression rate in cells transfected with Control and RNase L siRNA in Figure 2c).
OAS expression is impaired in human myotubes from OB-IR subjects and activation of RNase L with exogenous 2-5A allows to restore insulin response in OB-IR myotubes
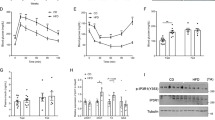
As stated in Introduction, not all obese people develop IR. Our results indicating that RNase L/RLI could impact insulin response in muscle cells, prompted us to analyze their expression in primary myotubes from obese subjects divided into two groups, insulin-sensitive (OB-IS) and insulin-resistant (OB-IR), on the basis of their homeostasis model assessment of IR (HOMAIR) index (Table 1). Accordingly with previous studies,38, 39 insulin-stimulated P-Akt/Akt expression rate and glucose uptake level were significantly reduced, by 56% (Figure 3a; **P=0.005) and 31% (Figure 3b; **P=0.003), respectively, in OB-IR group compared with OB-IS. In an unexpected way, RNase L and RLI levels were similar in the two groups of obese subjects (Figure 3c).
Insulin response and RNase L and RLI expression in insulin-sensitive and insulin-resistant obese subjects’ myotubes. (a) Differentiated myotubes from OB-IS (n=7) and OB-IR (n=8) subjects were incubated or not with insulin. P-Akt and Akt proteins expression was assessed on total cellular extracts by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. Data are expressed relative to the fold induction of P-Akt/Akt level in OB-IS subjects after insulin stimulation, which was set at 100%. **P<0.01. (b) Glucose uptake was measured in myotubes of OB-IS (n=7) and OB-IR (n=8) subjects. Differentiated cells were incubated or not with insulin then with 6-NBDG in a glucose-free medium. Fluorescence intensity was measured at 540 nm wavelength. Each bar of the histogram represents the ratio of fluorescence measured in basal condition (without insulin) and after incubation with insulin. **P<0.01. (c) Differentiated myotubes of OB-IS (n=7) and OB-IR (n=8) subjects were collected and expression of RNase L and RLI proteins was assessed on total cellular extracts by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. The mean value of OB-IS group was set at 100%
However, OAS2 protein level was decreased by 69% in myotubes of OB-IR group compared with OB-IS (Figure 4a; *P=0.045). We also evaluated OAS1 protein expression but failed to detect it. Moreover, OAS mRNA levels were also diminished in OB-IR group (Figure 4b; *P=0.05). However, contrary to OAS2 mRNA, the inter-subject variability did not allow for demonstrating any significant difference in OAS1 mRNA expression between the two groups. We hypothesized that the decreased levels of OAS mRNA in OB-IR myotubes could be due to an increase in the suppressor of cytokine signaling 3 (SOCS3) expression, which is induced during inflammation and is known as an important negative regulator of OAS expression40 and insulin response in muscle.41, 42 However, we found no difference in SOCS3 expression between OB-IS and OB-IR groups (Figure 4c).
OAS and SOCS3 expression and insulin response in insulin-sensitive and insulin-resistant obese subjects’ myotubes after 2-5A transfection. (a) Differentiated myotubes of OB-IS (n=7) and OB-IR (n=8) subjects were collected and expression of OAS2 protein was assessed on total cellular extracts by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. The mean value of OB-IS group was set at 100%. *P<0.05. (b) Expression of OAS1 and OAS2 mRNA was measured by q-PCR in myotubes of OB-IS (n=7) and OB-IR (n=7) subjects. For each mRNA, the mean level of expression in OB-IS group was set at 100%. *P≤0.05. (c) SOCS3 protein expression was assessed on total cellular extracts from myotubes of OB-IS (n=7) and OB-IR (n=8) subjects, by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. The mean value of OB-IS group was set at 100%. (d) Myotubes of OB-IS (n=7) and OB-IR (n=8) subjects were transfected or not with 2-5A and were then stimulated or not with insulin. P-Akt and Akt proteins expression was assessed on total cellular extracts by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. Data are expressed relative to the percentage of induction of P-Akt/Akt protein level in insulin-stimulated non-transfected OB-IS myotubes, which was set at 100%. In the histogram, insulin-stimulated P-Akt/Akt levels have been represented in 2-5A-transfected and non-transfected myotubes. *P<0.05 and **P<0.01. (e) Glucose uptake was measured in myotubes of OB-IS (n=7) and OB-IR (n=8) subjects. Differentiated cells were transfected or not with 2-5A, then incubated or not with insulin and finally with 6-NBDG in a glucose-free medium. Fluorescence intensity was measured at 540 nm wavelength. Each bar of the histogram represents the ratio of fluorescence measured in basal condition (without insulin) and after incubation with insulin. **P<0.01. (f) Myotubes of OB-IS (n=7) and OB-IR (n=7) subjects were transfected or not with 2-5A and expression of OAS1 and OAS2 mRNA was then measured by q-PCR. For each mRNA, the mean level of expression in non-transfected OB-IS myotubes was set at 100%. *P≤0.05
As OAS is responsible for 2-5A synthesis which is necessary for RNase L activation, we transfected OB-IS and OB-IR myotubes with an exogenous 2-5A. In the presence of 2-5A, insulin-stimulated P-Akt/Akt expression rate and glucose uptake level were significantly increased in OB-IR group, by 119% (Figure 4d; *P=0.013) and 38% (Figure 4e; **P=0.006), respectively, whereas it had no effect in OB-IS. Moreover, insulin-stimulated P-Akt/Akt and glucose uptake levels, which were significantly different between OB-IS and OB-IR groups, were no more significantly different between non-transfected OB-IS and 2-5A-transfected OB-IR myotubes, indicating that RNase L activation by exogenous 2-5A restored insulin response in OB-IR myotubes (Figures 4d and e). Important to note, 2-5A transfection also induced an increase of OAS1 and OAS2 mRNA expression in OB-IR myotubes, by 560 and 575%, respectively (Figure 4f; *P=0.047), that cancelled out the significance of the difference in OAS2 mRNA expression between OB-IS and OB-IR groups.
RNase L regulates neither PKR and IκB expression, nor JNK and IRS1 (serine 312) phosphorylation
PKR has an important role in blunting insulin signaling during inflammation via IRS1 phosphorylation on serine residues, directly or indirectly by phosphorylating and activating JNK.8, 9 Moreover, PKR also regulates IKK activation, leading to IκB degradation and NF-κB activation.10 On the other hand, it has been demonstrated that RNase L modulates PKR expression.43 However, we observed no difference in IκB, PKR, P-JNK/JNK and Ser312P-IRS1/IRS1 expression levels between OB-IS and OB-IR groups (Figure 5).
Analysis of PKR and IκBα expression and JNK and IRS1 phosphorylation in obese insulin-sensitive and insulin-resistant subjects’ myotubes. Myotubes of OB-IS (n=7) and OB-IR (n=8) subjects were collected and expression of IκBα, PKR, P-JNK, JNK, Ser312P-IRS1 and IRS1 proteins was assessed on total cellular extracts by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. For representation of IκBα and PKR expression levels and P-JNK/JNK and P-IRS1/IRS1 expression rates, the mean value of OB-IS group was set at 100%
RNase L regulates MnSOD expression
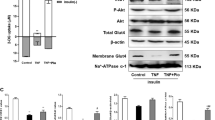
During obesity, lipid overload causes oxidative stress by increasing ROS production and/or altering anti-oxidant defenses that can lead to muscle IR.32 We thus checked in OB-IS and OB-IR myotubes mRNA expression of several major anti-oxidant enzymes and of the transcriptional nuclear factor erythroid 2-related factor 2 (NRF2) that regulates their expression. Only MnSOD mRNA was significantly underexpressed, by 77%, in myotubes of OB-IR subjects compared with OB-IS (Figure 6a; *P=0.021), which was confirmed at the protein level with 66% underexpression (Figure 6b, see white bars; **P=0.005). Furthermore, RNase L activation with exogenous 2-5A significantly increased MnSOD mRNA expression, by 285% (Figure 6c; **P=0.004), as well as MnSOD protein expression, by 176% (Figure 6b; *P=0.033), in OB-IR myotubes, allowing to cancel out the significances between OB-IS and OB-IR groups. As we observed for OAS1 and OAS2 mRNA (Figure 4f), 2-5A transfection increased MnSOD mRNA level in OB-IS myotubes, this effect being here statistically significant. We assume that, even when MnSOD mRNA is highly expressed, overactivation of RNase L by exogenous 2-5A could lead to its induction. However, the level of MnSOD protein was not modified after 2-5A transfection in OB-IS myotubes as it was in OB-IR myotubes (Figure 6b), which could be indicative of a regulation of MnSOD expression by a post-transcriptional mechanism.
Analysis of anti-oxidant enzymes and mitochondrial proteins expression in obese insulin-sensitive and insulin-resistant subjects’ myotubes. (a) Differentiated myotubes of OB-IS (n=7) and OB-IR (n=7) subjects were collected and expression of GPx 1, 2 and 4, copper/zinc SOD (Cu/ZnSOD), MnSOD, catalase and NRF2 mRNA was measured by q-PCR. For each mRNA, the mean level of expression in OB-IS group was set at 100%. *P<0.05. (b) Myotubes of OB-IS (n=8) and OB-IR (n=7) subjects were transfected or not with 2-5A and MnSOD protein expression was assessed on total cellular extracts, by western blotting. Membrane scans obtained from two different subjects of each group are presented in the figure. The mean level of MnSOD expression in OB-IS group, without 2-5A treatment, was set at 100%. *P<0.05 and **P<0.01. (c) After 2-5A transfection, expression of MnSOD mRNA was measured by q-PCR in myotubes of OB-IS (n=7) and OB-IR (n=8) subjects. The mean level of expression in non-transfected OB-IS myotubes was set at 100%. *P<0.05 and **P<0.01. (d) COX IV and UCP3 proteins expression was assessed on total cellular extracts from myotubes of OB-IS (n=7) and OB-IR (n=8) subjects, by western blotting. Membrane scans obtained from four different subjects of each group are presented in the figure. The mean value of OB-IS group was set at 100%. (e) Expression of TLR3 and TLR4 mRNA was measured by q-PCR in myotubes of OB-IS (n=7) and OB-IR (n=8) subjects. The mean level of expression in non-transfected OB-IS myotubes was set at 100%. *P<0.05. (f) Myotubes of OB-IS (n=7) and OB-IR (n=7) subjects were transfected or not with 2-5A and TLR3 mRNA expression was then measured by q-PCR. The mean level of expression in non-transfected OB-IS myotubes was set at 100%. *P<0.05
MnSOD is the mitochondrial SOD; however, its low expression in OB-IR myotubes does not seem due to a different mitochondrial mass between OB-IS and OB-IR myotubes, as we observed no variation in the expression of two other mitochondrial proteins: the mitochondrial uncoupling protein 3 (UCP3) and the subunit IV of cytochrome c oxidase (COX IV) (Figure 6d).
TLR3 expression is decreased in OB-IR myotubes
As RNase L activation by exogenous 2-5A restored the expression of MnSOD and OAS in OB-IR myotubes, we concluded that a common regulator of these proteins was affected in OB-IR myotubes contrary to OB-IS. An essential regulatory mechanism of MnSOD and OAS expression during inflammation and oxidative stress involves TLR3 activation. Indeed, previous studies have shown that TLR3 activation (i) protects cells against oxidative stress through the upregulation of MnSOD44, 45 and (ii) induces IFNI production and OAS expression after TLR3 recognition of small mRNA fragments. We thus measured TLR mRNA expression in OB-IS and OB-IR myotubes: unlike TLR4, TLR3 mRNA was significantly underexpressed, by 72%, in OB-IR myotubes compared with OB-IS (Figure 6e; *P=0.048). Finally, as OAS2 and MnSOD mRNA, 2-5A transfection induced a significant increase, by 195%, of TLR3 mRNA expression in OB-IR myotubes (Figure 6f; *P=0.031), allowing to cancel out the significance between OB-IS and OB-IR groups.
Discussion
Obesity has an undeniable impact on health by promoting the occurrence of chronic diseases including T2D.1 Although it is known that IR appears several decades before the onset of T2D, the pathophysiological mechanisms involved in the development of the disease are still not fully understood. Importantly, it is difficult to explain why a subset (30%) of obese individuals seems preserved from IR and other metabolic disorders during weight gain.2, 3 The identification of the pathways implicated in the regulation of insulin response in these two groups of obese people is a key issue to understand the pathogenesis of IR.
Skeletal muscle, due to its mass, is an essential organ in maintenance of glycemic homeostasis and has a key role in IR and T2D pathogenesis.31 So, understanding the cellular mechanisms that regulate insulin response in muscle of obese people is of primary importance and could help to understand the differences between insulin-sensitive and insulin-resistant subjects.
During the last decade, the central role played by innate immunity and TLR activation in the pathogenesis of IR has been demonstrated. RNase L, which is an essential component of the innate immune response, is activated through IFNI production following TLR 3/4 activation and could amplify TLR activation.20, 21, 22 RNase L activity is regulated by its binding to 2-5A, which is synthesized by OAS, and by RLI. We recently demonstrated that insulin response was impaired in RNase L−/−-MEF and partially restored after re-introduction of RNase L expression in these cells.29 These findings incited us to study the potential role of RNase L/RLI in regulation of insulin response, at muscle level.
Here, our results reveal that RNase L activity allows for maintaining an insulin response during FFA-induced inflammation in mouse and human myotubes. Indeed, in our two models, the impairment of insulin response induced by palmitate treatment, which mimics inflammation observed during obesity, was limited in cells expressing higher level of RNase L/RLI ratio (C2-RNaseL+ and Myo-siRLI) compared with respective control cells. Insulin-stimulated phosphorylation of Akt was even improved by RLI siRNA transfection in palmitate-treated human myotubes, showing the importance of RNase L activity to limit FFA deleterious effect on insulin response.
As stated above, one intriguing and still not explained fact is the existence of insulin-sensitive individuals among obese people. If RNase L and RLI levels were similar in two groups of obese individuals with different insulin sensitivity states, then the levels of OAS2 protein and OAS1 and OAS2 mRNA were lower in OB-IR myotubes. 2-5A level could thus be the limiting factor for a correct insulin response. Indeed, 2-5A transfection restored insulin-stimulated P-Akt/Akt and glucose uptake levels in OB-IR myotubes to comparable levels of those in OB-IS myotubes. These results confirm that low 2-5A production, due to decreased expression of OAS, leads to underactivation of RNase L and impaired insulin response in OB-IR myotubes. Our findings underline the importance of RNase L activity in the development of IR in skeletal muscle during obesity.
The next objectives of this study were to determine how RNase L can regulate insulin response and why OAS levels are low in OB-IR myotubes. One of the known targets of RNase L is another mediator of innate immunity, the enzyme PKR.43 PKR is activated in tissues of obese mice and, consequently, can directly or indirectly (through JNK activation) induce negative phosphorylation of IRS1 as well as activation of IKK. As a result, these two activated pathways lead to the disruption of insulin response combined with the synthesis of inflammatory cytokines.8, 9 However, we neither found any significant variation of PKR and IκB expression levels, nor of P-JNK/JNK and Ser312P-IRS1/IRS1 expression rates between OB-IS and OB-IR myotubes. According to these observations, the mechanism by which RNase L activity allows for maintaining insulin response in myotubes during obesity does not depend on PKR regulation.
As oxidative stress is induced during obesity and has an important role in IR development, we checked the expression levels of several enzymes implicated in the anti-oxidant defense. Only MnSOD was weakly expressed in OB-IR myotubes, that does not seem related to a lower mitochondria mass, as attested by the similar levels of two other mitochondrial proteins (UCP3 and COX IV) in OB-IS and OB-IR myotubes. MnSOD expression is necessary to maintain insulin sensitivity during obesity. Indeed, not only its expression is upregulated by treatments that improve insulin sensitivity,46, 47 but muscle transfection with plasmid allowing for increased MnSOD expression in vivo preserves insulin response during high-fat diet-induced obesity.48 We hypothesize that the high MnSOD expression observed in OB-IS myotubes may (i) counteract the highly toxic superoxide anion (O2•−) produced in excess by mitochondria during lipid overload32 and (ii) rise H2O2 production that is essential to maintain insulin sensitivity.49, 50 As an explanation of MnSOD underexpression in OB-IR myotubes, we identified a defect in TLR3 expression, which is known to protect against inflammation and oxidative stress in different models.44, 51, 52, 53, 54 The concomitant significant increase in MnSOD and TLR3 expression following 2-5A transfection would be in favor of our hypothesis.
We propose that, during FFA-induced inflammation, RNase L is activated by the OAS-synthesized 2-5A. RNase L then cleaves mRNA in smaller RNA, which in turn could activate TLR3 pathway. This activation induces the expression of the anti-oxidant enzyme MnSOD and numerous ISG among which the OAS genes. This secondary activation of OAS by cleaved mRNA entails increased 2-5A production that all the more activates RNase L. It then creates an amplification loop that leads to higher activation of TLR3 and higher levels of OAS and MnSOD, as observed in myotubes from OB-IS subjects. By controlling H2O2 level, MnSOD allows for maintaining Akt phosphorylation and glucose uptake, even when excessive ROS are produced. At the contrary, myotubes of OB-IR subjects express very low level of TLR3 that prevents this amplification loop to setting up. The consecutive underexpression of OAS and MnSOD thus causes the inhibition of insulin response in myotubes of OB-IR subjects. The global mechanism we identified here is recapitulated by a scheme, in Figure 7.
Role of RNase L and TLR3 activation during inflammation in insulin response of obese people’s myotubes. During obesity, chronic lipid overload triggers the development of global inflammation and oxidative stress, which are particularly deleterious for metabolic tissues such as skeletal muscle. RNase L, which is activated by the 2-5A synthesized by the OAS, cleaves mRNA, which in turn could activate TLR3 pathway. This activation allows the expression of MnSOD and of numerous ISG among which the OAS genes. The secondary activation of the OAS induced by cleaved mRNA leads to increased 2-5A production that all the more activates RNase L. It then creates an amplification loop that induces high levels of OAS and MnSOD expression in myotubes from insulin-sensitive obese people. By controlling H2O2 levels, high expression of MnSOD allows for maintaining Akt phosphorylation and glucose uptake, even when excessive ROS are produced. At the contrary, myotubes from obese insulin-resistant people express very low level of TLR3 that prevents this amplification loop to setting up. The consecutive underexpression of OAS and MnSOD is thus responsible for oxidative stress development and inhibition of insulin response. IR, insulin receptor; MyD88, myeloid differentiation primary response gene 88
Our results highlight that TLR3 signaling and RNase L/RLI have a critical role in the pathogenesis of obesity-associated IR. Importantly, for the first time, we identify a molecular mechanism induced by inflammation that could regulate anti-oxidant defenses and be responsible for the different insulin-sensitivity states observed among obese people, at skeletal muscle level. Going further into this pathway, in particular through the identification of regulatory factors of TLR3 expression, could lead to the determination of central mechanisms regulating insulin response during obesity and the discovery of new therapeutic targets.
Materials and Methods
Mouse myogenic cells
For conditional expression of RNase L, human complementary DNA (cDNA) was cloned in the LacSwitch II inducible mammalian expression system (Stratagene, Massy, France). The C2C12 clone in which RNase L is conditionally expressed (C2-RNaseL) and C2-RLI cells that overexpress RLI were previously described.28, 30 For induction of RNase L (C2-RNaseL+), differentiated C2-RNaseL cells were incubated with isopropyl-β-D-thiogalactopyranoside (IPTG) (2 mM) during 6 h.
Human cells
Primary human skeletal muscle cells from five normal-weight (19<body mass index (BMI)<25 kg/m2) subjects were provided by the Agence Française contre les Myopathies (AFM), in accordance with the French and European legislations.
The present study involved 15 non-diabetic obese (BMI>30 kg/m2) subjects, all with no personal or familial history of diabetes. Their HOMAIR index, which is frequently used in routine clinical medicine to estimate insulin sensitivity state, was evaluated to separate them into insulin-sensitive (OB-IS: HOMAIR<2) and insulin-resistant (OB-IR: HOMAIR>3) groups (a subject whom the HOMAIR index is superior to 3 is conventionally considered as insulin-resistant). The characteristics of these subjects are presented in Table 1. Skeletal muscle cells were obtained from biopsies in the vastus lateralis.55 This experimental protocol was approved by the local Ethic Committee (03/10/GESE, Montpellier, France) and informed written consent was obtained from all the participants.
Myoblasts isolation, purification and culture were performed as described by Barro et al.55 Cells between passages 2 and 6 were used for all the experiments.
Cell differentiation
At confluence, C2C12-derived cells and human myoblasts were switched to muscle differentiation medium (Dulbecco’s modified eagle medium (DMEM; Lonza, Basel, Switzerland) supplemented with 2% (V/V) FCS (PAN Biotech, Dutscher, Brumath, France)) for 5 days.
Cell transfection
Human cells were transfected 4 days after induction of muscle differentiation. siRNA were transfected with HiPerFect (Qiagen, Courtabœuf, France) following the manufacturer’s instructions. Cells were incubated with siRNA for 8 h at 37°C. siRNA sequences are indicated in Supplementary Table 1.
A non-stabilized non-modified 2-5A3 was enzymatically produced with double-stranded RNA-activated OAS from IFNβ-treated HeLa cells (500 U/ml)56 and was transfected at 10 nM final concentration with the Calcium Phosphate Transfection Kit (Sigma-Aldrich, Saint-Quentin Fallavier, France) following the manufacturer’s instructions. Cells were incubated for 8 h at 37°C with 2-5A then total RNA were extracted as described in a following paragraph. RNase L activation by 2-5A was checked by controlling 28S and 18S specific cleavage as previously described57, 58 (data not shown).
Induction of inflammation and insulin treatment
Four days after induction of differentiation, muscle cells were treated with palmitate, then insulin-stimulated and harvested. Lipid-containing media were prepared by conjugation of palmitate with FFA-free BSA (molar ratio palmitate/BSA ∼2.5).59 Cells were incubated for 16 h at 37°C with 750 μM palmitate. For insulin response, cells were treated with human insulin (1 nM) for 10 min at 37°C.
Western-blot assay
Differentiated cells were washed and lysed in SDS-PAGE sample buffer (300 mM Tris (pH 8.9), 5% (W/V) SDS, 750 mM β-mercaptoethanol, 20% (V/V) glycerol, bromophenol blue). From these cellular extracts, proteins were fractionated, electrophoretically transferred onto nitrocellulose membrane to be then incubated with the different antibodies and analyzed with the Odyssey Infrared Imaging System LI-COR (Biosciences, ScienceTec, Courtabœuf, France) as previously described.29 We used primary antibodies against the following proteins: Phospho-Akt (Ser473), Akt, IκBα, PKR, Phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, IRS1, SOCS3, COX IV (nine from Cell Signaling, Ozyme, Saint-Quentin Yvelines, France), RNase L (mouse), ABCE1 (mouse), OAS2 (three from Santa Cruz, Tebu-Bio, Le Perray en Yvelines, France), α-tubulin, UCP3 (both from Sigma-Aldrich), RNase L (human), Phospho-IRS1 (Ser312) (from Abcam, Paris, France), ABCE1 (human) (Abnova, Tebu-Bio) and MnSOD (Enzo Life Sciences, Villeurbanne, France). We used secondary antibodies conjugated to IRDye800 (Rockland, Tebu-Bio). α-Tubulin protein level was measured in each sample as an indicator of proteins quantity loading. Proteins levels were then quantified from membranes scans with the ImageJ software (http://rsbweb.nih.gov.gate2.inist.fr/ij/index.html) and corrected with the corresponding α-tubulin levels.
Glucose uptake
After 10 min incubation at 37°C with or without 1 nM insulin, differentiated human cells were incubated for 30 min at 37°C with 300 μM 6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-glucose (6-NBDG, Molecular Probes, Thermo Fisher Scientific, Illkirch Graffenstaden, France) in a glucose-free medium (Invitrogen, Life Technologies, Saint Aubin, France), as previously described.29 Fluorescence intensity of 6-NBDG was measured at 540 nm wavelength (465 nm excitation wavelength) with an Infinite 200PRO TECAN (Lyon, France). Fluorescence values were normalized by cell number.
Reverse transcription and real-time quantitative PCR
Total RNA were isolated using TRIzol (Invitrogen). cDNA were generated by reverse transcription with the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. To quantify genes expression, cDNA were used as templates in SYBR Green I Master real-time quantitative PCR (q-PCR) assays on LightCycler480 (Roche Diagnostics, Meylan, France). Cycle number was 40. Sample data were analyzed according to the comparative cycle threshold method and were normalized by stable reference gene of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Gene sequences for primer design were obtained from the NCBI Reference Sequences database. Primers were chosen using the Primer3 and LightCycler Probe Design (Roche) softwares. Forward and reverse primers were designed on different exon sequences when possible. Primers sequences are provided in Supplementary Table 2.
Statistical analysis
Values represented in the graphs are means; error bars refer to the standard errors of the mean (S.E.M.). Statistical analyses were performed using the SigmaStat software (RITME Informatique, Paris, France). Statistical significance was determined by using a one-way analysis of variance (ANOVA) followed up with all pairwise multiple comparison procedures (Holm–Sidak method) to compare values from two different groups, or by a paired t-test or a Wilcoxon signed-rank test (if normality failed) to compare values from the same group before and after treatment. Two groups were considered as statistically significantly different for P≤0.05.
Abbreviations
- ABCE1:
-
ATP-binding cassette sub-family E member 1
- CHOP10:
-
C/EBP-homologous protein 10
- COX IV:
-
cytochrome c oxidase
- Cu/ZnSOD:
-
copper/zinc superoxide dismutase
- C/EBP:
-
CCAAT-enhancer-binding protein
- DMEM:
-
Dulbecco’s modified eagle medium
- FFA:
-
free fatty acid
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- GPx:
-
glutathione peroxidase
- IFNI:
-
type I interferons
- IκB:
-
inhibitor of κB
- IKK:
-
IκB kinase
- IL:
-
interleukin
- IPTG:
-
isopropyl-β-D-thiogalactopyranoside
- IRS1:
-
insulin receptor substrate 1
- ISG:
-
interferon-stimulated gene
- H2O2:
-
hydrogen peroxide
- HOMAIR:
-
homeostasis model assessment of insulin resistance
- JNK:
-
c-Jun N-terminal kinase
- MAD5:
-
melanoma differentiation-associated gene 5
- MCP-1:
-
monocyte chemoattractant protein-1
- MnSOD:
-
manganese superoxide dismutase
- MyD88:
-
myeloid differentiation primary response gene 88
- 6-NBDG:
-
6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-glucose
- NF-κB:
-
nuclear factor κB
- NRF2:
-
nuclear factor erythroid 2-related factor 2
- OAS:
-
oligoadenylate synthetase
- PKB:
-
protein kinase B
- PKR:
-
double-stranded RNA-dependent protein kinase
- q-PCR:
-
real-time quantitative PCR
- RIG-1:
-
retinoic acid-inducible gene-1
- RLI:
-
RNase L inhibitor
- RNase L:
-
latent endoribonuclease
- ROS:
-
reactive oxygen species
- SAPK:
-
stress-activated protein kinase
- S.E.M.:
-
standard error of the mean
- SOCS3:
-
suppressor of cytokine signaling 3
- TLR:
-
toll-like receptor
- TNFα:
-
tumor necrosis factor α
- UCP3:
-
uncoupling protein 3
References
Kahn SE, Hull RL, Utzschneider KM . Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846.
Bluher M . Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes 2012; 19: 341–346.
Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005; 90: 4145–4150.
Bisbal C, Lambert K, Avignon A . Antioxidants and glucose metabolism disorders. Curr Opin Clin Nutr Metab Care 2010; 13: 439–446.
Gregor MF, Hotamisligil GS . Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29: 415–445.
Boden G . Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011; 18: 139–143.
Glass CK, Olefsky JM . Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012; 15: 635–645.
Carvalho-Filho MA, Carvalho BM, Oliveira AG, Guadagnini D, Ueno M, Dias MM et al. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology 2012; 153: 5261–5274.
Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 2010; 140: 338–348.
Kang R, Tang D . PKR-dependent inflammatory signals. Sci Signal 2010; 5: pe47.
Dasu MR, Jialal I . Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011; 300: E145–E154.
Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S et al. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol 2009; 29: 1370–1375.
Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH . Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009; 284: 27384–27392.
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A . Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 2009; 89: 27–71.
Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H . Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 2005; 7: 1021–1031.
Szypowska AA, Burgering BM . The peroxide dilemma: opposing and mediating insulin action. Antioxid Redox Signal 2011; 15: 219–232.
Bloch-Damti A, Potashnik R, Gual P, Le Marchand-Brustel Y, Tanti JF, Rudich A et al. Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia 2006; 49: 2463–2473.
Vinayagamoorthi R, Bobby Z, Sridhar MG . Antioxidants preserve redox balance and inhibit c-Jun-N-terminal kinase pathway while improving insulin signaling in fat-fed rats: evidence for the role of oxidative stress on IRS-1 serine phosphorylation and insulin resistance. J Endocrinol 2008; 197: 287–296.
Choubey D, Moudgil KD . Interferons in autoimmune and inflammatory diseases: regulation and roles. J Interferon Cytokine Res 2011; 31: 857–865.
Bisbal C, Silverman RH . Diverse functions of RNase L and implications in pathology. Biochimie 2007; 89: 789–798.
Malathi K, Dong B, Gale M Jr., Silverman RH . Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 2007; 448: 816–819.
Li XL, Ezelle HJ, Kang TJ, Zhang L, Shirey KA, Harro J et al. An essential role for the antiviral endoribonuclease, RNase-L, in antibacterial immunity. Proc Natl Acad Sci USA 2008; 105: 20816–20821.
Le Roy F, Salehzada T, Bisbal C, Dougherty JP, Peltz SW . A newly discovered function for RNase L in regulating translation termination. Nat Struct Mol Biol 2005; 12: 505–512.
Kariko K, Ni H, Capodici J, Lamphier M, Weissman D . mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 2004; 279: 12542–12550.
Takeda K, Akira S . TLR signaling pathways. Semin Immunol 2004; 16: 3–9.
Khoo JJ, Forster S, Mansell A . Toll-like receptors as interferon-regulated genes and their role in disease. J Interferon Cytokine Res 2011; 31: 13–25.
Bisbal C, Martinand C, Silhol M, Lebleu B, Salehzada T . Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J Biol Chem 1995; 270: 13308–13317.
Bisbal C, Silhol M, Laubenthal H, Kaluza T, Carnac G, Milligan L et al. The 2′-5′ oligoadenylate/RNase L/RNase L inhibitor pathway regulates both MyoD mRNA stability and muscle cell differentiation. Mol Cell Biol 2000; 20: 4959–4969.
Fabre O, Salehzada T, Lambert K, Boo Seok Y, Zhou A, Mercier J et al. RNase L controls terminal adipocyte differentiation, lipids storage and insulin sensitivity via CHOP10 mRNA regulation. Cell Death Differ 2012; 19: 1470–1481.
Salehzada T, Cambier L, Vu Thi N, Manchon L, Regnier L, Bisbal C . Endoribonuclease L (RNase L) regulates the myogenic and adipogenic potential of myogenic cells. PLoS ONE 2009; 4: e7563.
Stump CS, Henriksen EJ, Wei Y, Sowers JR . The metabolic syndrome: role of skeletal muscle metabolism. Ann Med 2006; 38: 389–402.
Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis 2012; 11: 30.
Ragheb R, Shanab GM, Medhat AM, Seoudi DM, Adeli K, Fantus IG . Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun 2009; 389: 211–216.
Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56: 1986–1998.
Li XL, Andersen JB, Ezelle HJ, Wilson GM, Hassel BA . Post-transcriptional regulation of RNase-L expression is mediated by the 3′-untranslated region of its mRNA. J Biol Chem 2007; 282: 7950–7960.
Zhou A, Molinaro RJ, Malathi K, Silverman RH . Mapping of the human RNASEL promoter and expression in cancer and normal cells. J Interferon Cytokine Res 2005; 25: 595–603.
Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS . Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol 2005; 15: 331–341.
Jackson S, Bagstaff SM, Lynn S, Yeaman SJ, Turnbull DM, Walker M . Decreased insulin responsiveness of glucose uptake in cultured human skeletal muscle cells from insulin-resistant nondiabetic relatives of type 2 diabetic families. Diabetes 2000; 49: 1169–1177.
Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H . Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 1998; 47: 1281–1286.
Vlotides G, Sorensen AS, Kopp F, Zitzmann K, Cengic N, Brand S et al. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun 2004; 320: 1007–1014.
Jorgensen SB, O'Neill HM, Sylow L, Honeyman J, Hewitt KA, Palanivel R et al. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes 2013; 62: 56–64.
Yang Z, Hulver M, McMillan RP, Cai L, Kershaw EE, Yu L et al. Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS ONE 2012; 7: e47493.
Khabar KS, Siddiqui YM, al-Zoghaibi F, al-Haj L, Dhalla M, Zhou A et al. RNase L mediates transient control of the interferon response through modulation of the double-stranded RNA-dependent protein kinase PKR. J Biol Chem 2003; 278: 20124–20132.
Borysiewicz E, Doppalapudi S, Kirschman LT, Konat GW . TLR3 ligation protects human astrocytes against oxidative stress. J Neuroimmunol 2013; 255: 54–59.
Rakkola R, Matikainen S, Nyman TA . Proteome analysis of human macrophages reveals the upregulation of manganese-containing superoxide dismutase after toll-like receptor activation. Proteomics 2007; 7: 378–384.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D . Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010; 12: 662–667.
Samjoo IA, Safdar A, Hamadeh MJ, Raha S, Tarnopolsky MA . The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr Diabetes 2013; 3: e88.
Boden MJ, Brandon AE, Tid-Ang JD, Preston E, Wilks D, Stuart E et al. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am J Physiol Endocrinol Metab 2012; 303: E798–E805.
McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA 2004; 101: 8852–8857.
Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 2009; 106: 8665–8670.
Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM . Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 2006; 53: 688–695.
Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci USA 2011; 108: 2372–2377.
Holfeld J, Tepekoylu C, Kozaryn R, Urbschat A, Zacharowski K, Grimm M et al. Shockwave therapy differentially stimulates endothelial cells: implications on the control of inflammation via Toll-like receptor 3. Inflammation 2013; 37: 65–70.
Patel AK, Hackam AS . Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol 2013; 54: 122–131.
Barro M, Carnac G, Flavier S, Mercier J, Vassetzky Y, Laoudj-Chenivesse D . Myoblasts from affected and non-affected FSHD muscles exhibit morphological differentiation defects. J Cell Mol Med 2010; 14: 275–289.
Bayard B, Bisbal C, Silhol M, Cnockaert J, Huez G, Lebleu B . Increased stability and antiviral activity of 2'-O-phosphoglyceryl derivatives of (2′-5′)oligo(adenylate). Eur J Biochem 1984; 142: 291–298.
Rusch L, Zhou A, Silverman RH . Caspase-dependent apoptosis by 2',5'-oligoadenylate activation of RNase L is enhanced by IFN-beta. J Interferon Cytokine Res 2000; 20: 1091–1100.
Wreschner DH, James TC, Silverman RH, Kerr IM . Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res 1981; 9: 1571–1581.
Wang X, Yu W, Nawaz A, Guan F, Sun S, Wang C . Palmitate induced insulin resistance by PKCtheta-dependent activation of mTOR/S6K pathway in C2C12 myotubes. Exp Clin Endocrinol Diabetes 2010; 118: 657–661.
Acknowledgements
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM), Université de Montpellier 1 (UM1) and Centre Hospitalier Régional Universitaire (CHRU) Montpellier. OF and CA were recipient of a Ministère de l'Enseignement Supérieur et de la Recherche (MESR) fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Federici
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Fabre, O., Breuker, C., Amouzou, C. et al. Defects in TLR3 expression and RNase L activation lead to decreased MnSOD expression and insulin resistance in muscle cells of obese people. Cell Death Dis 5, e1136 (2014). https://doi.org/10.1038/cddis.2014.104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2014.104
Keywords
This article is cited by
-
Human serum RNase-L level is inversely associated with metabolic syndrome and age
Cardiovascular Diabetology (2017)
-
RNASET2 is required for ROS propagation during oxidative stress-mediated cell death
Cell Death & Differentiation (2016)
-
The interplay between inflammation and metabolism in rheumatoid arthritis
Cell Death & Disease (2015)
-
Identification of co-expression gene networks, regulatory genes and pathways for obesity based on adipose tissue RNA Sequencing in a porcine model
BMC Medical Genomics (2014)
-
Koschei the immortal and anti-aging drugs
Cell Death & Disease (2014)