Abstract
Apoptosis has an essential role in controlling T cell homeostasis, especially during the contraction phase of an immune response. However, its contribution to the balance between effector and regulatory populations remains unclear. We found that Rag1−/− hosts repopulated with Bim−/− conventional CD4+ T cells (Tconv) resulted in a larger induced regulatory T cell (iTreg) population than mice given wild-type (WT) Tconv. This appears to be due to an increased survival advantage of iTregs compared with activated Tconv in the absence of Bim. Downregulation of Bcl-2 expression and upregulation of Bim expression were more dramatic in WT iTregs than activated Tconv in the absence of IL-2 in vitro. The iTregs generated following Tconv reconstitution of Rag1−/− hosts exhibited lower Bcl-2 expression and higher Bim/Bcl-2 ratio than Tconv, which indicates that iTregs were in an apoptosis-prone state in vivo. A significant proportion of the peripheral iTreg pool exhibits low Bcl-2 expression indicating increased sensitivity to apoptosis, which may be a general characteristic of certain Treg subpopulations. In summary, our data suggest that iTregs and Tconv differ in their sensitivity to apoptotic stimuli due to their altered ratio of Bim/Bcl-2 expression. Modulating the apoptosis pathway may provide novel therapeutic approaches to alter the balance between effector T cells and Tregs.
Similar content being viewed by others
Main
Regulatory T cells (Tregs) are an important subpopulation of CD4+ T cells that have a critical role in maintaining immune homeostasis and tolerance. Tregs can be divided into natural Tregs (nTreg), that develop in the thymus, and induced Tregs (iTreg), that develop from conventional CD4+ T cells (Tconv) in the periphery during an immune response. iTregs can be induced in response to either increased expression of self-antigen or as a consequence of infection. Therefore, their homeostasis must be tightly controlled. Insufficient differentiation of iTregs may result in immune pathogenesis, whereas prolonged survival of these cells may compromise the establishment of immune memory. Although significant insight has been obtained concerning Treg development and differentiation,1 little is known about the molecular mechanism(s) controlling nTreg and iTreg survival.2, 3, 4, 5
The BH3-only family member Bim (encoded by Bcl2l11) is a pro-apoptotic molecule that controls the coordinated contraction of the immune T cell response.6, 7, 8, 9 It directly interacts with and deactivates the anti-apoptotic regulators Bcl-2 and Bcl-xl. Thus, the fine balance between Bim and Bcl-2/Bcl-xl expression levels represents an important checkpoint for cell survival.10, 11 Despite enhanced peripheral T cell expansion, Bim−/− mice do not develop aggressive T cell-mediated autoimmune disease.12 This may be due in part to an increased Treg population in moderately aged Bim−/− animals compared with their wild-type (WT) littermates.13, 14 Interestingly, Bim expression naturally declines in peripheral WT CD4+ T cells during aging,15 which is co-incident with increasing numbers of Tregs. This raises the possibility that Bim has an important role in Treg homeostasis.
As iTregs and nTregs cannot be distinguished in Bim−/− animals, the impact that Bim depletion has on either Treg cell subtype remains unclear. It appears that depletion of Bim may have a limited impact on nTreg development, as the emigration rate of nTregs from the thymus is similar in WT and Bim−/− mice.13 The observation that Bim−/− nTregs are more proliferative is, however, contradictory to the observation that Bim−/− T cells are hypo-responsive upon TCR activation.16 In this study, we ask if the balance between Bim and Bcl-2 acts as a critical survival parameter that controls iTreg homeostasis.
Results
Preferential induction of iTregs in vivo in the absence of Bim
To directly investigate the influence of Bim depletion on iTreg homeostasis in vivo, we induced inflammatory bowel disease (IBD) in mice by adoptively transferring CD4+CD25−CD45RBhi Tconv into Rag1−/− hosts. In this model, transferred Tconv repopulate the host lymphoid tissue, leading to intestinal inflammation in a certain percentage of Tconv recipients. As only Tconv are transferred (Supplementary Figure S1a), essentially all Foxp3+ T cells recovered from the recipients are de novo-generated iTregs. Although Bim is important for T-cell apoptosis, we only observed a slight difference between Bim−/− and WT Tconv recipients in both percentage and absolute number of CD4+ T cells recovered from the spleens and mesenteric lymph nodes (MLN) (Figures 1a and b). Surprisingly, there was a significant increase in the percentage (∼2–3-fold) and number (∼3–4-fold) of Foxp3+CD4+ iTregs recovered from Bim−/− Tconv recipients compared with WT Tconv recipients in the spleen, MLN and a combined population of intraepithelial lymphocytes and lamina propria lymphocytes (IEL/LPL) isolated from the intestines (Figures 1c and d). This increase of iTregs did not appear to be due to differing levels of Foxp3 protein expression, as iTregs recovered from both Bim−/− and WT Tconv recipients exhibited comparable levels of Foxp3 expression (spleen=1022/958 (WT/Bim−/− MFI); MLN=1649/1443; intestine=1272/1258; Figure 1e). Nor was the difference due to an increased ability of Bim−/− Tconv to expand, compared with WT Tconv following transfer into a lymphopenic host, as the percentage of BrdU uptake by CD4+Foxp3− cells was similar in the spleen and surprisingly higher in WT cells in the MLN (Figure 1f and Supplementary Figure S1b). This difference, however, was minimal in terms of the number of CD4+Foxp3−BrdU+ cells (Supplementary Figure S1c). Interestingly, there was an increased percentage and number of Bim−/− CD4+Foxp3+BrdU+ cells in the spleen (Figure 1f and Supplementary Figure S1c). This may partially explain the larger fold difference between the number of Bim−/− iTregs and WT iTregs in the spleen (∼3-fold difference) compared with MLN (∼2-fold difference).
Enhanced in vivo iTreg induction and decreased incidence of IBD in the absence of Bim. Tconv were sorted from the spleen and lymph nodes of C57BL/6 (WT) or Bim−/− mice and injected i.v. into the tail veins of Rag1−/− recipients (0.4 × 106 cells per mouse). Four weeks post-transfer, spleen, MLN and IEL/LPL (intestine) were analyzed by flow cytometry. Percentage and number of CD4+ (a and b) and CD4+Foxp3+ (c and d) cells. Data are the mean±S.E. of two to three individual experiments with five to seven mice per group (a−d), and statistical analysis performed using standard unpaired t-test (*P<0.05, **P<0.01). (e) Representative dot plots of CD4+Foxp3+ iTregs. (f) Percentage of BrdU+ Tconv (CD4+Foxp3−, left) and iTregs (CD4+Foxp3+, right). Data are the mean±S.E. of two independent experiments with four mice per group, and statistical analysis performed using standard unpaired t-test (*P<0.05, **P<0.01). (g) Incidence of IBD in Tconv recipients and statistical summary (2 × 2 χ2-analysis)
Consistent with these data, mice that received Bim−/− Tconv had an ∼50% reduction in the incidence of IBD (as measured by weight loss greater than 5% initial body weight) than WT Tconv recipients (P=7.52 × 10−5; relative risk=0.491; 95% CI (0.346–0.700); Figure 1g). Taken together, these data suggest that the loss of Bim has a preferential effect on the generation and/or survival of iTregs, compared with the proportion of transferred Tconv that do not convert to a Foxp3+ population, resulting in a protective effect against IBD.
Loss of Bim confers a preferential survival advantage for iTregs
The increase in iTregs observed in the Bim−/− Tconv recipients could be a consequence of increased efficiency of the Bim−/− iTreg conversion, a selective survival advantage of Bim−/− iTregs due to increased IL-2 secretion by Bim−/− Tconv, or an accumulated effect due to an intrinsic survival advantage in iTregs versus Tconv in the absence of Bim. To address the first possibility, we performed an in vitro iTreg conversion assay. Multiple factors are able to modulate the efficiency of iTreg conversion. TCR signaling is known to have a pivotal effect. Enhanced TCR activation with higher proliferative capacity leads to more efficient iTreg differentiation in vitro (Supplementary Figures S2a and b) and in vivo.17 As recent studies have suggested that Bim−/− Tconv are hyporeactive to TCR stimulation,16 one might predict that Bim−/− Tconv may not show a differentiation advantage over WT Tconv. Consistent with this notion, Bim−/− Tconv exhibited less efficient iTreg conversion in vitro than WT Tconv (Supplementary Figure S2c), which was likely due to less efficient TCR activation. It is possible that Bim−/− Tconv secrete more IL-2, leading to enhanced conversion and survival of iTregs. However, this did not seem to be likely as WT and Bim−/− Tconv activated in vitro secrete similar amounts of IL-2 (Supplementary Figure S2d). Furthermore, the percentage of CD4+CD25−IL-2+ cells isolated from Rag1−/− mice that received either WT or Bim−/− Tconv was equivalent (Supplementary Figure S2e).
The third possibility is that Bim depletion has a differential effect on the survival of iTregs versus Tconv. Constitutive expression of the IL-2 receptor (CD25) is a hallmark for nTregs and it is well known that they are highly dependent on IL-2 for their homeostasis and survival.18, 19 Given the fact that Bim is a principal component in growth factor deprivation-mediated apoptosis, we compared the survival capacity of WT and Bim−/− iTregs, and activated Tconv in the absence of IL-2. The iTregs were generated by activating purified Tconv from Foxp3GFP reporter mice in the presence of TGF-β and IL-2 for 3 days. Foxp3+ iTregs (GFP+) and activated Foxp3− Tconv (GFP−) were sorted, cultured in the absence of exogenous IL-2 and their relative survival compared. We found that Foxp3+ iTregs had a two-fold increase in their sensitivity to apoptosis in cultures without the addition of exogenous IL-2, compared with Foxp3−-activated Tconv (Figures 2a and b). Foxp3+ iTregs were more vulnerable to apoptosis than Foxp3−-activated Tconv over a wide range of limited exogenous IL-2 supplementation (from 10 U/ml to 100 U/ml; Figure 2c). However, these differences in survival capacity between iTregs and activated Tconv were abrogated in both cell populations in the absence of Bim (Figures 2a and b). These data suggest that iTregs are more sensitive to IL-2 deprivation-mediated apoptosis than activated Tconv. Loss of Bim expression confers a greater survival advantage for Foxp3+ iTregs, nullifying their increased sensitivity to growth factor withdrawal.
Preferential role of the Bim/Bcl-2 axis in controlling iTreg survival. (a and b) Tconv were isolated from WT or Bim−/− Foxp3GFP mice and activated with anti-CD3/CD28-coated beads in the presence of TGF-β (5 ng/ml) and IL-2 (100 U/ml) for 3 days to induce iTreg differentiation. The iTregs (GFP+) and activated Tconv (GFP−) were then sorted by FACS and cultured for additional 3 days in the absence of IL-2. Percentage of live iTregs and Tconv was determined by flow cytometry with propidium iodide (PI) and anti-Annexin V. Data are the mean±S.E. of four independent experiments with statistical analysis performed using standard paired t-test (*P<0.05). (c) iTregs (GFP+) and activated Tconv (GFP−) were cultured in the presence of various IL-2 concentrations for 3 days and percentage of live cells determined by PI and anti-Annexin V. Data is representative of two independent experiments
Intrinsic vulnerability to apoptosis due to altered Bim, Bcl-2 and Bcl-xl balance in iTregs
We next investigated the mechanism responsible for the survival disparity between iTreg and activated Tconv in the presence of limited IL-2. Activated T cells only express IL-2 transiently following TCR engagement. Blocking IL-2 signaling by using an anti-CD25 antibody had little effect on activated Tconv survival in the absence of exogenous IL-2 addition. Thus, the survival advantage of activated Tconv over iTregs under limiting IL-2 conditions was likely due to cell intrinsic mechanisms rather than an autocrine IL-2 pathway.
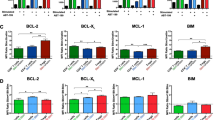
As deletion of the pro-apoptotic molecule Bim was able to mitigate the survival differences between iTregs and activated Tconv, we compared the expression levels of several pro- and anti-apoptotic Bcl-2 family members in iTregs and activated Tconv before (pre) and after (post) a 2-day culture in the absence of exogenous IL-2 (Figure 3a). The expression of the pro-apoptotic members of the Bcl-2 family, Bim and Bax, was increased to a similar extent in iTregs and activated Tconv (change between expression pre- and post-IL-2 removal). No change in Bid expression level was observed. Expression of the anti-apoptotic molecules Bcl-2 and Bcl-xl was slightly higher in iTregs compared with activated Tconv before IL-2 withdrawal (Figure 3a; noted as ‘pre’). However, culturing cells in the absence of exogenous IL-2 for just 48 h resulted in a much more dramatic decrease of Bcl-2 expression in iTregs compared with activated Tconv, which was accompanied with a larger increase of Bim expression (Figure 3a; noted as ‘post’). Similar but less pronounced changes were observed with Bcl-xl and Bax. Mcl-1 and Noxa have recently been shown to be important in regulating T-cell apoptosis during clonal expansion.20 Despite substantial upregulation of Noxa following T-cell activation, there was no difference in the relative expression of Mcl-1 and Noxa mRNA between Tconv and iTregs (Supplementary Figure S3).
Intrinsic vulnerability of Bim, Bcl-2 and Bcl-xl balance in iTregs. (a and b) iTregs and activated Tconv were prepared as in Figure 2. mRNA was isolated from either freshly sorted iTregs (Foxp3+) and activated Tconv (Foxp3−), before (pre) or after an additional 48 h of culture in the absence of IL-2 (post). Expression levels of genes indicated was assessed by qPCR. Data shown are the mean±S.E. from five independent experiments with statistical analysis performed using standard paired t-test (*P<0.05, **P<0.01, ***P<0.005). (c) Intracellular Bcl-2 of freshly sorted iTregs (Foxp3+) and activated Tconv (Foxp3−) before (pre) or after an additional 48 h of culture in the absence of IL-2 (post). Data represent two independent experiments
As cell survival is dependent on the balance between pro- and anti-apoptotic molecules rather than their absolute expression levels, analysis of the relative ratios of interactive partners may be an informative parameter to elucidate cellular sensitivity to apoptosis. Minimal differences in the Bim/Bcl-2, Bim/Bcl-xl, Bax/Bcl-2 and Bax/Bcl-xl expression ratios were observed between freshly sorted iTregs and activated Tconv (Figure 3b; pre), suggesting that their initial sensitivity to apoptosis may be similar. However, continued culture in the absence of IL-2 resulted in a significant increase in the Bim/Bcl-2 expression ratio in iTregs (∼7-fold – pre versus post) versus activated Tconv (∼3-fold). There was also an ∼3-fold increase in the Bim/Bcl-xl ratio in iTregs, which was not observed with activated Tconv (Figure 3b). Similar changes were observed with the Bax/Bcl-2 or Bax/Bcl-xl ratios. As anticipated, IL-2 supplementation prevented the increase of Bim/Bcl-2 ratio, which correlated with their capacity to promote iTreg survival to a level comparable to activated Tconv (Supplementary Figure S4). Consistent with this mRNA analysis, we observed a much larger proportion of iTregs that lost intracellular Bcl-2 protein expression following IL-2 withdrawal, compared with activated Tconv (Figure 3c). Collectively, these data suggest that iTregs are intrinsically less capable of maintaining an appropriate balance between the pro- (Bim/Bax) and anti- (Bcl-2/Bcl-xl) apoptotic proteins to ensure survival.
Reduced Bcl-2 expression in iTregs in vivo
To extend our findings in vivo, we transferred the Foxp3GFP Tconv or CD4+CD25−CD45RBhi Tconv from WT mice into Rag1−/− recipients and analyzed the expression levels of the Bcl-2 family molecules in iTregs and Tconv recovered 4 weeks post-transfer. Although expression levels of Bim, Bcl-xl and Bax were fairly comparable between iTreg and Tconv, iTregs consistently expressed four to five times less Bcl-2 than Tconv recovered from the spleen (Figure 4a). Consequently, the Bim/Bcl-2 and Bax/Bcl-2 mRNA ratios were substantially higher in iTregs than in Tconv (Figures 4b and c). No difference in Bax/Bcl-xl ratio was observed (Figure 4c). Interestingly, Bcl-2 mRNA expression in iTregs and Tconv recovered from the MLN was similar, which mirrored our observation that there was a somewhat less profound increase in the number of iTregs in MLN versus the spleen in the absence of Bim (Figures 1c and d). Given that the MLN serves as the primary site of immune response in the IBD model, it is possible that the levels of growth factors such as IL-2 are more abundant here compared with more distal lymphoid tissues such as the spleen.
Reduced Bcl-2 expression in iTregs in vivo. Foxp3GFP Tconv or CD4+CD25−CD45RBhi cells from WT or Bim−/− mice (0.5 × 106) were transferred into Rag1−/− hosts. (a–c) Four weeks post transfer, iTregs (GFP+ cells) and Tconv (GFP− cells) were isolated by FACS, mRNA prepared, and expression of the genes indicated was determined by qPCR. Data shown are representative of three independent experiments with statistical analysis performed using the Mann–Whitney test (*P<0.01). (d and e) Four weeks post-transfer, iTregs and Tconv from the spleen, non-draining lymph nodes (ndLN), MLN and IEL/LPL were analyzed for intracellular Bcl-2 and Foxp3 by flow cytometry. Data are mean±S.E. of three individual experiments with 11–12 individual mice, with statistical analysis performed using standard unpaired t-test comparing Tconv/iTreg and iTreg in the spleen/MLN (*P<0.05, ***P<0.005)
Intracellular Bcl-2 protein staining confirmed that iTregs consistently express less Bcl-2 (∼3-fold) compared with Foxp3− Tconv isolated from the spleen, non-draining lymph nodes, MLN and IEL/LPL of repopulated Rag1−/− hosts (Figures 4d and e). This was also observed with cells isolated from Rag1−/− hosts repopulated with Bim−/− Tconv (Figure 4e). Bcl-2 expression in iTregs was also consistently higher in the MLN. Collectively, iTregs seem to have a deficiency in Bcl-2 expression, which may render them more susceptible to apoptosis in vivo.
Foxp3+Bcl2lo Treg populations in vivo
The nTregs are reported to express higher levels Bcl-2 than Tconv.21 If low expression of Bcl-2 is a characteristic feature of iTregs, in comparison with nTregs, we asked whether it could potentially mark iTregs in WT C57BL/6 mice in vivo. Consistent with our hypothesis, there were clearly two populations of Tregs, distinguishable as Bcl-2lo and Bcl-2hi in the spleen and peripheral lymph nodes, respectively (Figure 5a). Although Bcl-2 expression levels in Tregs did not correlate with Helios expression, a suggested marker of iTregs,22, 23 about 50% of the Bcl-2lo Tregs were CD69lo and CD44hi, suggesting that this Treg population was antigen-experienced but not recently activated (Figures 5a and b). Therefore, although we cannot exclude the possibility that a proportion of the Bcl-2lo Tregs were derived from nTregs after recent activation, it is possible that Foxp3+Bcl-2loCD69hiCD44hi cells represent newly differentiated iTregs, whereas Foxp3+Bcl-2loCD69loCD44hi cells represent a memory iTreg population.24 However, further analysis would be required to determine if nTregs and iTregs can be distinguished by the level of Bcl-2 expression.
Identification of Foxp3+Bcl2lo regulatory T-cell population in vivo. (a and b) Cells isolated from the peripheral lymph nodes or spleens were stained with Foxp3, Bcl-2 and surface markers indicated. (b) CD4+Foxp3+ cells were stained for Bcl-2, CD44 and CD69. Data represent analysis of five independent experiments
Discussion
Previous studies have reported increased numbers of Tregs in Bim−/− mice, as well as in aged mice.13, 14 However, the mechanisms that control the homeostasis of Treg populations remain to be fully defined. It has been suggested that increased proliferation of Bimlo Tregs (marked by increased BrdU incorporation in the Foxp3+Bimlo population) was the basis for preferential Treg expansion.13 However, this model is somewhat controversial as studies have suggested that responses following TCR stimulation are defective in Bim−/− and Bimlo T cells.15, 16 Our data suggest that decreased Bim expression confers a preferential survival advantage on iTregs. Therefore, depletion or downregulation of Bim limits the rapid peripheral Treg cell turnover,25 which consequently results in increased Treg numbers. As optimal TCR signaling is required for iTreg differentiation, and the T-cell population with the highest proliferative capacity tends to differentiate into iTregs more efficiently, higher BrdU incorporation in the Foxp3+Bimlo T-cell population may represent enhanced iTreg survival due to lower Bim expression in vivo rather than nTreg expansion. In line with our model, overexpression of the anti-apoptotic molecule Bcl-2 in T cells also results in an increased proportion of iTregs in vivo and reduces the incidence of autoimmune disease.26
The importance of IL-2 in the development and homeostatic maintenance of nTregs, iTreg and Tconv is well documented.18, 19, 27 IL-2- or IL-2R-deficient mice have a two-fold reduction in the number of Tregs and suffer from a severe lymphoproliferative disease.2, 28 However, crossing IL-2- or IL-2R-deficient mice with Bim−/− mice eliminated this numerical deficiency, supporting our data that demonstrates the level of vBim expression is important for iTreg survival.28 Nevertheless, restoration of Foxp3+ Treg numbers in Il2−/−Bim−/− mice did not prevent the lymphoproliferative disease unless exogenous IL-2 was added, suggesting that IL-2 is also required for the function of Tregs in vivo.28 It has been reported that IL-2 and signaling through the IL-2R regulates Bcl-2 expression.29 Our data supports this observation as IL-2 deprivation resulted in a significant decrease in both Bcl-2 and Bcl-xl expression in vitro. Taken together, this strongly suggests an IL-2-dependent link between Bim and Bcl-2 levels, and supports our conclusion that in the absence of Bim, there is increased survival of iTregs and consequently a higher number of Tregs. How this relates to a decrease in IBD incidence may be two-fold. First, in the absence of Bim, there may be a reduction in apoptosis resulting in more iTregs. Second, the increased number of Bim−/− iTregs results in a lower ratio of Tconv/iTregs in the tissues (spleen, WT –.86 : 1, Bim−/− – 22 : 1; MLN, WT – 31 : 1, Bim−/− – 20 : 1; Gut, WT – 303 : 1, Bim−/− – 120 : 1), which may increase the probability that Bim-deficient iTregs outcompete Tconv for the available IL-2 that is required for optimal Treg suppression in vivo.28
Although the notion that different T-cell subpopulations may preferentially use different pro- and anti-apoptotic pathways to regulate homeostasis has been previously proposed,30 no clear examples have been documented. Our data suggest that balanced expression within the Bim/Bcl-2 axis has a greater impact on the survival of iTregs than activated Tconv in vitro and in vivo. It is possible that lineage-specific transcription factors may preferentially modulate the expression of specific Bcl-2 family apoptotic modulator(s). A previous ChIP–ChIP study revealed that Foxp3 is constitutively bound to the Bcl-2 locus, but not any other Bcl-2 family members.31 It remains unclear whether such binding promotes or represses Bcl-2 expression. Although depletion of the Foxp3-recruited transcriptional repressor Eos results in increased Bcl-2 expression, iTregs themselves express more Bcl-2 than activated Tconv.21 A more intriguing hypothesis is that Foxp3 may modulate Bcl-2 expression in a conditional manner. By recruiting different nuclear factors, Foxp3 may promote Bcl-2 expression when IL-2 is in excess, but repress Bcl-2 expression when IL-2 is limited. This may be particularly relevant in specific diseases such as autoimmune diabetes, in which defective IL-2 production has been documented.32 If Foxp3 is directly modulating Bcl-2 expression in Tregs, it is unclear whether nTregs, like iTregs, are also hypersensitive to apoptosis.
A decreased Treg/Tconv cell ratio has been implicated in the onset of autoimmune diabetes.32 Indeed, consistent with our finding that iTregs have a greater apoptotic tendency in vitro and in vivo, profound Treg apoptosis has been shown to occur during diabetes onset.32 Interestingly, our study suggests that there may be two subpopulations of Tregs expressing different levels of Bcl-2, and it is the Bcl-2hi population that is preferentially lost during progression towards diabetes. The majority of the detectable Tregs in islets are phenotypically CD4+Foxp3+Bcl-2loKi67+, which may represent newly differentiated iTregs at the site of inflammation. In summary, the balance between Tregs and Tconv has a central role in maintaining immune tolerance and promoting immune response. Perturbation of the Bim/Bcl-2 balance may represent a new therapeutic approach to modulate the immune environment under diverse disease conditions. Modulation of Bcl-2 expression could be used as an approach to promote survival of iTregs generated for therapeutic application.
Materials and Methods
Mice, T-cell preparation and antibodies
C57BL/6 (WT) and Rag1−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The Bim−/− mice were provided by Andreas Strasser (The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia).12 The Bim−/− mice were crossed with Foxp3GFP reporter mice2 to generate Bim−/−Foxp3GFP mice. All mice were bred and maintained at St. Jude Children's Research Hospital. All animal experiments were performed in the American Association for the Accreditation of Laboratory Animal Care-accredited, specific pathogen-, Helicobacter- and MNV-free facilities, following national, state and institutional guidelines. Animal protocols were approved by the St. Jude Animal Care and Use Committee.
For IEL/LPL purification, intestines were removed, cut into 0.5 cm pieces and shaken at 37°C for 20 min in 45 ml HBSS with 1 mM DTT. Suspension was decanted and filtered through a 70 μm cell strainer into a 50 ml conical tube, and tissue was pelleted by centrifugation for 5 min at 1200 r.p.m. Supernatant was discarded and pellet was reserved on ice. This process was repeated two more times with the strained tissue, pelleting the suspension in the same 50 ml conical tube. The remaining intestinal tissue was finely chopped and incubated in 25 ml RPMI supplemented with 10% FBS and 8000 U of Collagenase Type IV (Worthington Biochemical, Lakewood, NJ, USA), rotating at 37°C. Cell suspension was strained and pooled with the previous pelleted collection, centrifuged and supernatant discarded. This was repeated with the strained intestinal tissue. The final cell pellet was washed in RPMI/10% FBS, centrifuged and supernatant discarded. The cell pellet was resuspended in 4 ml of 40 × percoll (4.0 ml of 100 × percoll+6.0 ml RPMI/10% FBS; GE Healthcare, Piscataway, NJ, USA) and strained through a 70 μm cell strainer. Cell suspension was gently layered over 5.0 ml of 100 × percoll in a 15 ml conical tube and centrifuged at 2500 r.p.m. with no brake for 20 min at room temperature. Media overlying the band of IEL/LPL at the interface between the two percoll layers was carefully removed. The band of cells was transferred by pipette to a new 15 ml conical tube to which RPMI/10% FBS was added to a final volume of 10 ml. The tube was shaken and centrifuged 10 min at 1500 r.p.m. to pellet the cells. For Tconv and Treg preparation, spleens and lymph nodes from mice were processed and stained with fluorochrome-conjugated antibodies and purified by single-cell sorting using anti-CD4 (clone GK 1.5), anti-CD25 (clone PC61) and anti-CD45RB (clone C363-16A). Tconv used in this study were defined as CD4+CD45RBhiCD25−, or if the Foxp3GFP reporter mice were used, CD4+GFP−. All antibodies used were from Biolegend (San Diego, CA, USA) or eBiosciences (San Diego, CA, USA). Bcl-2 expression was determined using clone 10C4, and staining performed with Foxp3 fixation/permeablization reagents from eBioscience.
IBD model
Induction of IBD was performed as described previously.33 Briefly, 0.4 × 106 FACS-purified Tconv (CD4+CD45RBhiCD25−) were injected via the tail vein into Rag1−/− mice. The Tconv recipients were monitored and the clinical sign of disease was defined as 5–10% body-weight loss. Spleen, lymph node and IEL/LPL cellularity was analyzed approximately 4 weeks post-Tconv transfer. For ex vivo analysis of gene expression pattern, iTregs and Tconv were sorted 4 weeks post-transfer.
Analysis of IL-2 by ELISA and intracellular staining
WT and Bim−/− Tconv were sorted and plated in 96-well round-bottom plates (1 × 105 per well) with anti-CD3/CD28-coated M-450 tosylactivated dynabeads (Invitrogen, Grand Island, NY, USA) at a ratio of 2.5 cells per bead. After 72 h stimulation, supernatants were collected and analyzed by ELISA. Supernatants were incubated on plates pre-coated with 1 μg/ml purified anti-IL2 (clone JES6-1A12, Biolegend) for 2 h at room temperature. Then, IL-2 was detected with 1 μg/ml biotinylated anti-IL-2 (clone JES6-5H4 biotin, Biolegend), followed by streptavidin-HRP (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA) and developed with TMB substrate (Thermo Fisher Scientific, Pittsburgh, PA, USA).
For intracellular staining, spleen and MLN of Rag1−/− that had received WT or Bim−/− Tconv 4 weeks prior were processed, and the cells were stimulated for 12 h with PMA/ionomycin (100 ng/ml and 500 ng/ml, respectively) in the presence of Brefeldin A (1 : 1000, Biolegend). Following stimulation, cells were stained for surface CD4 and CD25, fixed and stained with antibodies to IL-2 (clone JES5-5H4) or an IgG isotype control, using Cytofix/Cytoperm kit following the manufacturer's instruction (BD Biosciences, San Diego, CA, USA), and analyzed by flow cytometry.
Intracellular staining for Bcl-2 and Foxp3
Purified Tconv (CD4+CD45RBhiCD25−) were injected i.v. via the tail vein into Rag1−/− mice. After 4 weeks, spleen and MLN were processed and cells were stained for surface CD4, and then fixed, permeabilized and stained with antibodies to Bcl2 (clone BCL/10C4), Foxp3 (clone 150D) and an IgG1 isotype control, using the Foxp3 Fixation/Permeabilization buffer set from EBiosciences according to manufacturer's instructions, and analyzed by flow cytometry.
BrdU analysis
Purified Tconv were injected via the tail vein into Rag1−/− mice. At 4 weeks post-transfer, mice were injected i.p. with 200 μl of BrdU (10 mg/ml) in PBS. After 16 h, cells from spleen and MLN were harvested, stained for CD4, Foxp3 and BrdU, using the BrdU-APC flow kit (BD Biosciences) following manufacturer's instructions, and analyzed by flow cytometry.
Induced regulatory T-cell differentiation and survival analysis
Tconv were isolated from WT or Bim−/− Foxp3GFP reporter mice and activated with either plate-bound anti-CD3 (clone 2C11) and soluble anti-CD28 (clone 37.51) or pre-coated CD3/CD28 beads in the presence of 5 ng/ml TGF-β and 100 U/ml IL-2 for 3 days. GFP+ iTreg and activated GFP− Tconv were sorted by FACS. Either cell type was then cultured with or without exogenous IL-2 at a cell density of 5 × 104 per well in 96-well round-bottom plates for the times indicated. Samples were collected and stained with eFluor-670 (eBiosciences), AnnexinV (BD Biosciences) and PI (Sigma-Aldrich, St. Louis, MO, USA). Live cells were defined as AnnexinV−PI−.
Quantitative real-time PCR
RNA was purified using Qiagen (Valencia, CA, USA) RNeasy Plus Mini or RNeasy Micro kit following the manufacturer's instructions. The expression level of Bim, Bcl-2, Bcl-xl, Bax, Bid, Mcl1 and Noxa were quantified by real-time PCR on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR green. Taqman probe-based quantitative PCR was used to record actin expression level for normalization. Primer sets were as follows: bcl2-F: 5′-ACTTCGCAGAGATGTCCAGTCA-3′, bcl2-R: 5′-TGGCAAAGCGTCCCCTC-3′; bcl-xl-F: 5′-GAATGGAGCCACTGGCCA-3′, bcl-xl-R: 5′-GCTGCCATGGGAATCACCT-3′; bax-F: 5′-GCCTCCTCTCCTACTTCGGG-3′, bax-R: 5′-TGAGGACTCCAGCCACAAAGA-3′; Bim-F: 5′-TGCGCCCGGAGATACG-3′, Bim-R: 5′-TTCGTTGAACTCGTCTCCGA-3′; Bid-F: 5′-GCTGTCTCCCTATTTCCAGGTG-3′, Bid-R: 5′-AGCTGAACGCAGAGATGTCCA-3′; Noxa-F: 5′- GTCGCAAAAGAGCAGGATGAG-3′, Noxa-R: 5′-TTGAGCACACTCGTCCTTCAA-3′; Mcl-1F: 5′-AACGGGACTGGCTTGTCAAA-3′, Mcl-1R: 5′-CTAGGTCCTGTACGTGGAAGAACTC-3′.
Statistics
Data were analyzed using paired, unpaired t-test or Mann–Whitney test, depending upon the data set, and is described in the figure legends. For analysis of disease incidence in Figure 1f, a 2 × 2 χ2-analysis was used. P-values were categorized into three levels: *P<0.05, **P<0.01 and ***P<0.005, unless stated otherwise.
Abbreviations
- Tconv:
-
conventional T cell
- nTreg:
-
natural regulatory T cell
- iTreg:
-
induced regulatory T cell
- WT:
-
wild type
- IBD:
-
inflammatory bowel disease
- MLN:
-
mesenteric lymph nodes
- IEL/LPL:
-
intraepithelial lymphocytes and lamina propria lymphocytes
References
Sakaguchi S, Yamaguchi T, Nomura T, Ono M . Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787.
Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY . A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005; 6: 1142–1151.
Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B et al. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol 2005; 175: 32–36.
Rahman MK, Midtling EH, Svingen PA, Xiong Y, Bell MP, Tung J et al. The pathogen recognition receptor NOD2 regulates human FOXP3+ T cell survival. J Immunol 2010; 184: 7247–7256.
Singh N, Yamamoto M, Takami M, Seki Y, Takezaki M, Mellor AL et al. CD4(+)CD25(+) regulatory T cells resist a novel form of CD28- and Fas-dependent p53-induced T cell apoptosis. J Immunol 2010; 184: 94–104.
Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity 2002; 16: 759–767.
Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P . Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 2008; 28: 197–205.
Hutcheson J, Scatizzi JC, Siddiqui AM, Haines III GK, Wu T, Li QZ et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity 2008; 28: 206–217.
Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM . Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity 2008; 28: 218–230.
Willis SN, Adams JM . Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 2005; 17: 617–625.
Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med 2007; 204: 1665–1675.
Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738.
Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P et al. A major role for Bim in regulatory T cell homeostasis. J Immunol 2011; 186: 156–163.
Pandiyan P, Lenardo MJ . The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct 2008; 3: 6.
Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL . Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol 2010; 185: 4535–4544.
Ludwinski MW, Sun J, Hilliard B, Gong S, Xue F, Carmody RJ et al. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. J Clin Invest 2009; 119: 1706–1713.
Gottschalk RA, Corse E, Allison JP . TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med 2010; 207: 1701–1711.
Vignali DA, Collison LW, Workman CJ . How regulatory T cells work. Nat Rev Immunol 2008; 8: 523–532.
Cheng G, Yu A, Malek TR . T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 2011; 241: 63–76.
Wensveen FM, van Gisbergen KP, Derks IA, Gerlach C, Schumacher TN, van Lier RA et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity 2010; 32: 754–765.
Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 2009; 325: 1142–1146.
Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184: 3433–3441.
Akimova T, Beier UH, Wang L, Levine MH, Hancock WW . Helios expression is a marker of T cell activation and proliferation. PLoS One 2011; 6: e24226.
Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK . Response to self antigen imprints regulatory memory in tissues. Nature 2011; 480: 538–542.
Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest 2006; 116: 2423–2433.
Gonzalez J, Tamayo E, Santiuste I, Marquina R, Buelta L, Gonzalez-Gay MA et al. CD4+CD25+ T cell-dependent inhibition of autoimmunity in transgenic mice overexpressing human Bcl-2 in T lymphocytes. J Immunol 2007; 178: 2778–2786.
Davidson TS, DiPaolo RJ, Andersson J, Shevach EM . Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 2007; 178: 4022–4026.
Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 2010; 185: 6426–6430.
Miyazaki T, Liu ZJ, Kawahara A, Minami Y, Yamada K, Tsujimoto Y et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 1995; 81: 223–231.
Krammer PH, Arnold R, Lavrik IN . Life and death in peripheral T cells. Nat Rev Immunol 2007; 7: 532–542.
Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY . Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007; 445: 936–940.
Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008; 28: 687–697.
Izcue A, Coombes JL, Powrie F . Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev 2006; 212: 256–271.
Acknowledgements
We would like to thank Andreas Strasser for the knockout mice. We are also very grateful to Richard Cross, Greig Lennon, Stephanie Morgan and Jennifer Rogers for FACS; Karen Forbes, Ashley Castellaw and Amy Krause-McKenna for maintenance, breeding and genotyping of mouse colonies; Fabien Llambi for helpful discussions; and the staff of the St. Jude Animal Resource Center for the animal husbandry. This work was supported by the NIH (AI39480, AI091977), the St. Jude Cancer Center Support CORE Grant (CA21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by P Salomoni
Supplementary Information accompanies the paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, X., Szymczak-Workman, A., Gravano, D. et al. Preferential control of induced regulatory T cell homeostasis via a Bim/Bcl-2 axis. Cell Death Dis 3, e270 (2012). https://doi.org/10.1038/cddis.2012.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2012.9
Keywords
This article is cited by
-
Blockade of miR-142-3p promotes anti-apoptotic and suppressive function by inducing KDM6A-mediated H3K27me3 demethylation in induced regulatory T cells
Cell Death & Disease (2019)
-
Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells
Nature Immunology (2013)