Abstract
We have previously reported that the human p53 gene encodes at least nine different p53 isoforms, including Δ133p53α, which can modulate p53 transcriptional activity and apoptosis. In this study, we aimed to investigate the regulation of Δ133p53α isoform expression and its physiological role in modulating cell cycle arrest and apoptosis. We report here that in response to a low dose of doxorubicin (which induces cell cycle arrest without promoting apoptosis), p53 directly transactivates the human p53 internal promoter, inducing Δ133p53α protein expression. The induced Δ133p53α then inhibits p53-dependent apoptosis and G1 arrest without inhibiting p53-dependent G2 arrest. Therefore, endogenous Δ133p53α does not exclusively function in a dominant-negative manner toward p53, but differentially regulates cell cycle arrest and apoptosis.
Similar content being viewed by others
Main
The p53 tumor suppressor protein is a transcription factor that prevents damaged cells to proliferate in response to various cellular stresses.1, 2, 3 However, the ways by which p53 determines the cell fate outcome remain elusive.4 So far, the best described mechanism is its ability to modulate gene expression by binding directly and specifically to p53 responsive elements (p53REs) on DNA.5
We previously established that the human p53 gene expresses nine p53 protein isoforms (p53, p53β, p53γ, Δ40p53α, Δ40p53β, Δ40p53γ, Δ133p53α, Δ133p53β and Δ133p53γ) because of alternative splicing, alternative initiation of translation at codon 40 and alternative internal promoter. The Δ133p53α, Δ133p53β and Δ133p53γ isoforms are N-terminally truncated p53 protein isoform deleted of the first 132 aa, of which mRNA is initiated from the internal promoter in intron-4 of the human p53 gene.6, 7 p53 isoforms are expressed in normal human tissues and in tumors.8, 9, 10, 11, 12, 13, 14, 15
We have previously shown that ectopically expressed Δ133p53α negatively regulates p53 tumor suppressor activity by inhibiting p53-mediated apoptosis.6 However, little is known about the molecular mechanisms by which human Δ133p53α modulates p53 activities at physiological level.
In this study, we investigated the regulation of Δ133p53α expression and its physiological role in modulating the cellular response to DNA damage. We determined that endogenous Δ133p53α does not exclusively function in a dominant-negative manner toward p53 to regulate gene expression.
Results
Mapping of the p53 internal promoter within intron-4 of the human p53 gene
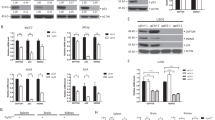
In the p53 homologous genes, p63 and p73, ΔN isoforms are transcribed from an internal promoter (P2), located within intron-3 of both genes.15 We previously established that human p53 gene contains an internal promoter located in the intron-4.6 To map the transcription initiation site of p53 internal promoter (1555 bp upstream of exon-5 of the human p53 gene), we generated different luciferase reporter constructs (Figure 1A), which were transfected into p53-null H1299 cells. As shown in Figure 1B, the 1555 bp fragment (pi3i4luc a) contains a promoter activity. Its activity is similar to the basal promoter activity of Bax and is approximately four times greater than the basal activity of the promoterless pGL3-basic plasmid. Deletion of residues 1–1304 (Figure 1A, constructs c, d and f) did not abolish the promoter activity (Figure 1B, c, d and f), suggesting that the minimum p53 internal promoter region is located within the last 251 bp of the intron-4 (Figure 1A, f).
Mapping of the promoter activity within intron-4 of the p53 gene. (A) Diagram showing the different p53 internal promoter deletion constructs (a–f), generated as described in Materials and Methods. Positions of the different restriction enzymes and size of the fragments obtained are indicated. Numbers of p53 introns and exons (empty and shadow boxes, respectively) are indicated. (B) Basal activity of each p53 internal promoter deletion construct (a–f). H1299 cells were co-transfected with each of the p53 internal promoter deletion constructs (a–f) and the Renilla luciferase reporter plasmid. The dual luciferase assay was then performed, as described in Materials and Methods. Each experiment was performed in duplicate and all results shown are the average of at least three separate individual experiments. The promoterless pGL3-basic plasmid was used as a negative control and pBax-luc plasmid was used as a positive control for the luciferase promoter activity. All activities were normalized to pGL3-basic activity
Moreover, the minimum p53 internal promoter (Figure 1B, f) showed a significant increase in the basal promoter activity (fourfold), as compared with the basal activity of the pi3i4-luc construct (Figure 1B, a), suggesting that the region 1–1304 bp contains negative regulatory elements such as silencers. In fact, the basal promoter activity of the construct f (1304–1555) is increased by threefold compared with the basal promoter activity of the construct d (1042–1555), suggesting that the region 1042–1304 contains a silencer element. Deletion of the last 251 bp from the 3′ end of intron-4 (Figure 1A, constructs b and e) completely abolishes the promoter activity (Figure 1B, b and e), confirming that the initiation site of the p53 internal promoter is located within the last 250 bp of intron-4 of the human p53 gene.
Human Δ133p53 is a direct p53 target gene
Using our bioinformatic software and our refined consensus p53RE,16 we identified a cluster of five p53 consensus decamers at the junction of exon-4 and intron-4 of the human p53 gene corresponding to the residues 757–804 in the pi3i4-luc construct (Figure 2A, dashed box).
Identification and characterization of five p53REs within the p53 internal promoter. (A) Diagram showing the identified p53REs (written in bold and in capital letters within the region of 757–804 bp). Constructs with (a, c) or without (d) the p53REs were generated, as described in Materials and Methods. p53 introns and exons (empty and shadow boxes, respectively) are indicated. 4i corresponds to intron-4. p53RE region is indicated with a dashed box. (B) Induction of the p53 internal promoter by p53. H1299 cells were co-transfected with each of the p53 internal promoter constructs (a, c and d) and the p53 expression vector or the empty expression vector pSI. The dual luciferase assay was then performed, each experiment was performed in duplicate and all results shown are the average of at least three separate individual experiments. The p53-inducible p21 promoter cloned upstream of the luciferase gene was used as a positive control (p21). All luciferase activities were normalized to the basal activity obtained with the empty pSI plasmid and are represented as fold activation. (C) Four single point mutations (underlined) were generated within the first two p53REs of the pi3i4-luc construct (A, a). WT p53RE and Mutant p53RE sequences are illustrated. H1299 were co-transfected with the pi3i4-luc construct (WT p53RE or Mutant p53RE) and the p53 expression vector or the empty expression vector PSI. The dual luciferase assay was then performed, each experiment was performed in duplicate and all results shown are the average of at least three separate individual experiments. All luciferase activities were normalized to the basal activity of the empty pSI plasmid and are represented as fold activation
To determine whether p53 directly transactivates its own internal promoter, deletion constructs c and d (Figure 1A) were assessed for their responsiveness to p53 (Figure 2A, c and d, respectively). A luciferase construct driven by the p21 promoter was used as a positive control. As shown in Figure 2B, p53 induced the luciferase activity in all constructs containing the putative p53RE (Figure 2A, a and c). Interestingly, the greatest induction (sixfold) was obtained with the construct containing the 723–1555 bp fragment (Figure 2A, construct c), which contains all five decamers, but lacks the upstream 722 bp region of the p53 internal promoter. This suggests that the region (1–722) contains several negative regulatory elements. Interestingly, deletion of the five putative decamers (Figure 2A, construct d) resulted in a significant decrease in p53 responsiveness (Figure 2B,d compared with c) without abolishing it, suggesting that p53 can induce p53 internal promoter through other transcription factors. Bioinformatics analysis revealed that the p53 internal promoter could be regulated by other transcription factors, including AP1, elk-1, N-myc, E2F, HIF-1α, HSF1 and ER (Supplementary Figure A).
To further confirm that the p53RE is functional, four single base mutations were generated by site-directed mutagenesis within the first two decamers of the internal promoter. As shown in Figure 2C, the four mutations are sufficient to significantly decrease p53 transactivation of the entire p53 internal promoter (construct a). This result clearly demonstrates that intron-4 of the p53 gene contains a functional p53RE. However, these mutations do not completely abolish p53 activation probably because of the presence of the remaining decamers. Further analysis will be required to establish whether the remaining decamers can confer responsiveness to p53.
Endogenous p53 can directly bind to and transactivate its internal promoter
We next investigated whether endogenous p53 could directly bind to and transactivate its own internal promoter. MCF7 cells (WTp53) were left untreated or treated with 60 ng/ml of actinomycin D (Act D) for 2 h and a chromatin immunoprecipitation (ChIP) assay was performed. The immunoprecipitated DNA was quantified by RT-qPCR (Figure 3a). A probe and primers specific for a region within the GAPDH gene that is not bound by p53, was used as a negative control. The binding of p53 to the p21 promoter was analyzed as a positive control (Supplementary Figure B1). We further quantified the genomic DNA co-immunoprecipitated with p53 protein and containing either the p53RE (intron-4) or exon-8 of the p53 gene. In unstressed cells (Figure 3a), no significant binding of p53 to either intron-4 or exon-8 was observed. However, following Act D treatment, endogenous p53 was bound to intron-4, but not to exon-8, demonstrating that p53 binds to its own internal promoter.
Human p53 internal promoter is directly transactivated by p53. (a) ChIP assay of p53 internal promoter DNA was carried out by RT-qPCR in MCF7 cells that were left untreated or treated with 60 ng/ml of actinomycin D (Act D) and harvested 2 h after treatment, using primers encompassing the p53REs. Exon-8 amplification was performed as a negative control. The amounts of the p53 internal promoter contained in the input or immunoprecipitated with the DO-1 antibody were quantified by RT-qPCR, as previously described.29 The results are expressed as a percentage of promoter specifically immunoprecipitated by the DO-1 antibody, compared with the total amount of promoter contained in 10% of the input. The results shown are the average of three independent experiments. (b–e) Quantification of endogenous p53 internal promoter activity by RT-qPCR in human cancer cells (MCF7, MCF7-DDp53, HCT116−/−, HCT116+/+ and U2OS) or primary normal human dermal fibroblasts (NHDF). (b) MCF7 (WTp53) and MCF7-DDp53 (mutant p53) cells were left untreated or treated for 30 min, 1 h or 2 h with 60 ng/ml of Act D and harvested. (c) HCT116+/+ (WTp53) and HCT116−/− (devoid of FLp53 expression) cells were left untreated or treated for 1 h with 0.5 μM of doxorubicin (Doxo) and harvested at the indicated time (d) U2OS (WTp53) cells and (e) NHDF were left untreated or treated for 1 h with 0.5 μM of doxorubicin (Doxo) and harvested 24 h after treatment. Student's t-test was performed and P-values are indicated (the student's t-tests were determined by comparing the expression levels of the p53 internal promoter in cells treated with Act D or doxorubicin, to untreated cells). **P<0.01; ***P<0.001. For all RT-qPCR experiments, expression levels were normalized to TBP. Results are expressed as the fold change compared with untreated cells and represent mean±S.D. of three independent experiments
RT-qPCR experiments were carried out in MCF7 and MCF7-DDp53 (devoid of functional p53) cells, to determine whether there is a p53-dependent induction of Δ133p53 mRNAs in response to Act D. (Figure 3b). We designed a probe and primers from the unique 5′UTR region of Δ133p53 mRNAs, encoding Δ133p53α, Δ133p53β and Δ133p53γ, to quantify the transcriptional activity of the p53 internal promoter. p21 mRNA expression was determined as a positive control (Supplementary Figure B2). In Figure 3b, the expression of Δ133p53 mRNAs is significantly induced 2 h following Act D treatment in MCF-7 cells, but no induction of Δ133p53 mRNAs was seen in MCF7-DDp53 cells, confirming that the induction of Δ133p53 mRNAs is p53-dependent.
To assess Δ133p53 mRNAs induction in other cell lines, we compared the expression of Δ133p53 mRNAs in response to doxorubicin treatment in colorectal carcinoma cell lines, expressing WTp53 (HCT116+/+) or devoid of full-length p53 (FLp53) expression (HCT116−/−). In HCT116−/− cells, the neomycin gene has been integrated within exon-2 of the p53 gene, resulting in the loss of FLp53 expression.17 However, HCT116−/− cells still express p53 isoforms at the mRNA and protein levels (Δ40p53 and Δ133p53 isoforms).7, 18 We show here that Δ133p53 mRNAs are inducible in response to doxorubicin only in HCT116+/+, but not in HCT116 −/− cells (Figure 3c). Similar results were obtained in response to etoposide treatment (data not shown). We further determined that Δ133p53 mRNAs is significantly induced on doxorubicin treatment in U2OS osteosarcoma cell line (WTp53) and primary normal human dermal fibroblasts (NHDF; Figure 3d and e, respectively). Altogether, these results demonstrate that in vivo, the p53 internal promoter located within intron-4, is directly transactivated by p53 in response to DNA damage in multiple cell lines.
Δ133p53α protein expression is induced by p53 in response to DNA damage
Western blot analyses were performed to determine whether the increase of Δ133p53α protein is associated with the expression of Δ133p53 mRNAs. The rabbit polyclonal antibody CM1 can detect all p53 protein isoforms, including Δ133p53α. We thus analyzed Δ133p53α protein expression in HCT116+/+ and HCT116−/− cells, treated or not with doxorubicin. To prevent any in vitro protein degradation, cells were directly lyzed in SDS-Laemmli buffer. Upon doxorubicin treatment, HCT116+/+ cells induces p53 and some p53 isoforms migrating at 48, 45 and 35 kDa (Figure 4a). In HCT116−/− cells, a strong expression of Δ40p53 was detected, but neither Δ40p53 nor other p53 isoforms were induced in response to doxorubicin treatment. To identify the bands corresponding to Δ133p53α, we designed two distinct small interfering RNAs (siRNAs; si133a and si133b), which specifically target the 5′UTR of Δ133p53 mRNA variants.
Δ133p53α protein is induced in response to doxorubicin treatment. (a) HCT116+/+ (p53WT) and HCT116−/− (devoid of FLp53 expression) cells were treated with 0.5 μM doxorubicin for 1 h and proteins were extracted 24 h after treatment in SDS-Laemmli buffer. Western blot analysis was performed using the CM1 rabbit polyclonal antibody, which recognizes all p53 isoforms. (b) HCT116+/+ transfected for 24 h with 50 nM of si133a, si133b (two distinct siRNAs specific for the 5′UTR of Δ133p53 mRNAs) or siNS (nonspecific siRNA used as a negative control), were left untreated (−) or treated (+) for 1 h with 0.5 μM of doxorubicin, as indicated. Proteins were extracted 24 h after treatment and analyzed by western blotting. p53 and its isoforms were revealed with the CM1 antibody. Actin was used as a protein loading control. (c) Western blot analysis of endogenous Δ133p53α expression in untreated (−) or doxorubicin-treated (+) NHDF cells. p53 and its isoforms were revealed with the CM1 antibody. Tubulin was used as a protein loading control
Having confirmed the specificity and efficiency of si133a or si133b (Supplementary Figure C1), we transfected HCT116 cells with si133a, si133b or non-specific siRNA (siNS) as a negative control (Figure 4b). Doxorubicin treatment induced the expression of p53 and also of some p53 isoforms migrating at 48, 45 and 35 kDa (Figure 4b lane 5 compared with 1). The two Δ133p53 siRNAs, si133a and si133b, did not alter the level of endogenous p53 or of the other p53 isoforms (corresponding to the bands at 48 and 45 kDa), but specifically reduced the expression of the 35 kDa band (Figure 4b, lanes 4, 6 compared with 5), indicating that the 35 kDa band corresponds to endogenous Δ133p53α protein.
The induction of Δ133p53α protein was further confirmed by western blot analysis in primary NHDF treated with doxorubicin (Figure 4c). Altogether, these results demonstrate that in the cancer derived cell lines (HCT116 WTp53) and in the normal primary fibroblasts, Δ133p53α isoform is induced by p53 at both mRNA and protein levels in response to DNA damage.
Generation and characterization of U2OS-ctrl and U2OS-Δ133p53 cells
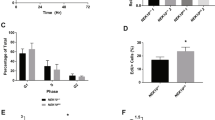
To determine the role of Δ133p53α in the cellular response to DNA damage, we investigated using bromodeoxyuridine (BrdU) cell cycle analysis and apoptosis assay (annexinV assay), the experimental conditions that trigger cell cycle arrest, but not apoptosis of U2OS cells in response to doxorubicin. We established that U2OS cells grown for 24 h at 50% confluence and treated with a low dose of doxorubicin (0.5 μM for 1 h), trigger G2 cell cycle arrest without promoting apoptosis 24 h after treatment. Those experimental conditions were then used in all the following experiments.
Two cellular models were generated to define the biological relevance of Δ133p53α induction in response to DNA damage. U2OS cells were stably transfected with the empty pcDNA3 or pcDNA3-Δ133p53α expression vectors, to generate U2OS-ctrl and U2OS-Δ133p53 cells, respectively.
First, we analyzed ectopic Δ133p53α protein expression in U2OS-Δ133p53 cells by western blot analysis, using the CM1 antibody (Figure 5A). U2OS-Δ133p53 cells transfected with si133a, si133b or siNS were left untreated or treated with doxorubicin (0.5 μM, 1 h). As shown in Figure 5A, lane 1, ectopic Δ133p53α protein was expressed at 35 kDa. A weaker band at 32 kDa could also be detected and may correspond to a post-translationally modified form of Δ133p53α. Transfection of U2OS-Δ133p53 cells with si133a or si133b specifically knock down the bands at 35 and 32 kDa corresponding to ectopic Δ133p53α protein, without affecting p53 protein level (Figure 5A, lanes 2, 3 compared with 1). In U2OS-Δ133p53 cells, doxorubicin treatment induced p53 protein expression, but had no detectable effect on Δ133p53α protein level (Figure 5A, lane 4 compared with 1). This is because the ectopic expression of Δ133p53α, driven by the CMV promoter (pcDNA3 plasmid), is exceeding the endogenous expression level and therefore masks the induction of endogenous Δ133p53α (Figure 5B lane 1 compared with 7). Depletion of Δ133p53α by si133a or si133b was equally efficient in doxorubicin-treated and untreated U2OS-Δ133p53 cells (Figure 5A, lanes 2, 3 compared with 1 and lanes 5, 6 compared with 4). The induction of p53 in response to doxorubicin is comparable in U2OS-Δ133p53 cells transfected with si133a, si133b or siNS (Figure 5A, lanes 5, 6 compared with 4). Therefore, p53 can be induced in U2OS-Δ133p53 cells in response to doxorubicin treatment.
Δ133p53α antagonizes DNA damage-induced apoptosis and G1 arrest without preventing p53-dependent G2 cell cycle arrest. U2OS-ctrl and U2OS-Δ133p53 cells were generated after stable transfection with the empty pcDNA3 or the pcDNA3-Δ133p53 expression vectors, respectively, as described in Material and Methods. Cells transfected for 24 h with 50 nM of si133a, si133b, siTA (siRNA specific for FLp53) or siNS (as indicated), were left untreated (−) or treated (+) for 1 h with 0.5 μM of doxorubicin (Doxo), and incubated for a further 24 h before harvesting. Western blot analysis of U2OS-Δ133p53 cells (A) and U2OS-ctrl cells (B). p53 and its isoforms were revealed with the CM1 antibody. Tubulin was used as a protein loading control. C (a–f) Cell cycle analysis by flow cytometry after BrdU pulse labeling of U2OS-ctrl and U2OS-Δ133p53 cells treated as described above. These experiments were carried out in parallel with the western blot analyses described above and a representative BrdU pulse labeling experiment is shown (C). The average of the percentage of cells in G1 (D) and G2 (E) are shown. (F) Apoptosis assay. In parallel to the western blot analyses described above, cells were treated as described above and Annexin V-FITC flow cytometric analysis was carried out. The average of the percentage of apoptotic cells is shown. Student's t-test was performed and P-values are indicated (the student's t-tests were carried out by comparing percentages of U2OS-ctrl cells transfected with si133a, si133b or siTA, to percentages of U2OS-ctrl cells transfected with siNS, after doxorubicin treatment). All results shown are the average of at least three separate individual experiments
To compare the expression level of Δ133p53α protein in U2OS-ctrl and U2OS-Δ133p53 cells, the same amount of protein from U2OS-Δ133p53 cells was loaded in Figure 5A, lane 1 and in Figure 5B, lane 1. Endogenous Δ133p53α protein was not detectable in untreated U2OS-ctrl cells (Figure 5B, lanes 2 and 3). Transfection of si133a or si133b did not affect the expression of p53 in untreated U2OS-ctrl cells (Figure 5B, lanes 4 and 5). In response to doxorubicin (0.5 μM, 1 h), endogenous Δ133p53α was induced in U2OS-ctrl cells (Figure 5B, lane 3 compared with 7). It is noteworthy that in U2OS-ctrl cells treated with doxorubicin, endogenous Δ133p53α protein is induced, but is far less expressed than ectopic Δ133p53α protein in U2OS-Δ133p53 cells (Figure 5B lane 7 compared with 1). Transfection of U2OS-ctrl cells with si133a or si133b inhibited the induction of endogenous Δ133p53α protein in response to doxorubicin (Figure 5B, lanes 8, 9 compared with 7). To determine whether the induction of Δ133p53α in doxorubicin-treated U2OS-ctrl cells is p53-dependent, cells were transfected with siTA, an siRNA that specifically targets p53 mRNAs containing exon-2 of the p53 gene without targeting Δ133p53 mRNAs. The results show that Δ133p53α protein induction is inhibited in doxorubicin-treated U2OS-ctrl cells depleted of p53 expression, confirming that Δ133p53α is induced by p53 (Figure 5B, lane 6 compared with 7).
Human Δ133p53α antagonizes p53-mediated apoptosis and G1 cell cycle arrest without preventing G2 cell cycle arrest
We next investigated whether the cellular response to DNA damage could be regulated by modulating Δ133p53α expression. U2OS-ctrl and U2OS-Δ133p53 cells were transfected with si133a, si133b or siNS and were left untreated or treated with doxorubicin (0.5 μM, 1 h) for 24 h. In parallel to the western blots presented in Figure 5A and B, cell cycle progression (Figure 5C–E) and apoptosis (Figure 5F) were also analyzed using BrdU assay and Annexin-V assay, respectively. A representative BrdU experiment is shown in Figure 5C. Untreated U2OS-ctrl and U2OS-Δ133p53 cells transfected with siNS showed similar cell cycle progression and a low percentage of spontaneous apoptosis (8%), indicating that overexpression of Δ133p53α does not alter cell cycle progression and apoptosis in untreated cells (Figure 5C-a, C-b, D, E and F).
U2OS-ctrl and U2OS-Δ133p53 cells transfected with siNS and treated with a low dose of doxorubicin show statistically similar cell cycle progression with about 3% of cells arrested in G1 and 79% of cells arrested in G2 (Figure 5C-c, C-d and E). In both cell lines, the low dose of doxorubicin did not significantly increase apoptosis compared with untreated cells (Figure 5F). Altogether, this indicates that overexpression of Δ133p53α in U2OS-Δ133p53 cells does not alter the cellular response to a low dose of doxorubicin, compared with U2OS-ctrl cells (Figure 5C-c, C-d, D, E and F). U2OS-ctrl cells were transfected with siTA, we detected a statistically significant decrease in the percentage of cells arrested in G2 (Figure 5E) and in the percentage of apoptotic cells, compared with doxorubicin-treated U2OS-ctrl cells transfected with siNS (Figure 5F). This is consistent with previous publications reporting that doxorubicin induces G2 cell cycle arrest and apoptosis in a p53-dependent manner in U2OS cells.19 As we have previously reported that overexpression of Δ133p53α can function in a dominant negative manner toward p53,6, 7 it is surprising that the overexpression of Δ133p53α in U2OS-Δ133p53 cells does not inhibit p53-mediated G2 arrest. It implies that Δ133p53α does not exclusively function in a dominant-negative manner toward p53.
Strikingly, in response to doxorubicin treatment, depletion of the Δ133p53 isoforms with si133a or si133b significantly enhanced the percentage of U2OS-ctrl cells in G1 arrest (16 and 26%, respectively, Figure 5C-e and D), suggesting that endogenous Δ133p53α inhibits G1 cell cycle arrest in U2OS-ctrl cells. To confirm this, U2OS cells overexpressing Δ133p53α (U2OS-Δ133p53) were transfected with si133a or si133b. Although both siRNAs inhibit Δ133p53α expression in U2OS-Δ133p53 cells, Δ133p53α protein level was still overexpressed compared with endogenous Δ133p53α protein level in U2OS-ctrl cells (Figure 5A). Transfection of si133a or si133b in U2OS-Δ133p53 does not significantly change the percentage of cells in G1 in response to treatment (Figure 5C-f and D), confirming that si133a and si133b are specific and that Δ133p53α inhibits G1 arrest in U2OS-ctrl cells.
Regarding apoptosis (Figure 5F), in U2OS-ctrl cells transfected with si133a or si133b and compared with cells transfected with siNS, a statistically significant increase in the percentage of apoptotic cells was detected, suggesting that a low dose of doxorubicin can trigger apoptosis if cells are depleted of Δ133p53α. Consistent with this, in U2OS-Δ133p53 cells transfected with si133a or si133b a statistically significant lower increase in the percentage of apoptotic cells was observed in response to treatment. This indicates that overexpression of Δ133p53α rescued the resistance to apoptosis in response to a low dose of doxorubicin confirming that endogenous Δ133p53α inhibits p53-mediated apoptosis in U2OS-ctrl cells.
Altogether, our results show that in response to a low dose of doxorubicin, endogenous Δ133p53α antagonizes p53-mediated apoptosis and G1 arrest, without inhibiting p53-dependent G2 arrest. This indicates that Δ133p53α does not exclusively inactivate p53. Moreover, it demonstrates that the cellular response to DNA damage could be regulated by modulating Δ133p53α expression.
Human Δ133p53α can form a protein complex with p53 and can differentially regulate p21, HDM2 and Bcl-2 expression in response to stress
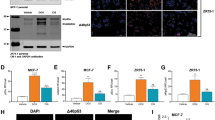
We have previously reported that ectopic Δ133p53α can act in a dominant-negative manner toward p53.7 To explain the regulation of p53 activities by Δ133p53α, we investigated whether Δ133p53α could directly interact with p53. Co-immunoprecipitation assays were carried out on p53-null H1299 cells transfected with Δ133p53α and/or p53, using the mouse monoclonal antibody DO-1, recognizing FLp53 but not Δ133p53α. Immunoprecipitated proteins were then analyzed by western blot with the rabbit polyclonal antibody (CM1), recognizing FLp53 and Δ133p53α. The results clearly show that Δ133p53α can form a protein complex with p53 (Figure 6a).
Δ133p53α directly interacts with p53 and differentially regulates p21, HDM2 and Bcl-2 expression. (a) Co-immunoprecipitation of Δ133p53α with p53. H1299 cells were transfected with pSV-p53 and/or pSV-Δ133p53α expression vectors, as indicated. Extracted proteins were immunoprecipitated with the mouse monoclonal antibody DO-1 or with a nonrelevant mouse monoclonal IgG antibody, as indicated. DO-1 is specific for FLp53 and does not bind Δ133p53α. Protein content was then analyzed by western blot analysis using the rabbit polyclonal antibody (CM1), recognizing FLp53 and Δ133p53α. 10% of the input was loaded as a control. (b) Western blot analysis of p53 target genes (p21, HDM2 and Bcl-2). In parallel to the cell cycle analysis and apoptosis assay, protein extracts from U2OS-ctrl cells treated as described in Figure 5B, were analyzed by western blotting using the corresponding antibodies. Actin was used as a protein loading control
We then studied whether endogenous Δ133p53α could regulate p53 target gene expression in response to DNA damage. In this study, we focused on a few p53 target genes (p21, HDM2 and Bcl-2), implicated in cell cycle progression and apoptosis. p21 encodes a cyclin-dependent kinase inhibitor involved in G1 and G2 cell cycle arrest,20 whereas HDM2 is an E3-ubiquitin ligase that regulates p53 protein degradation.21, 22 Both p21 and HDM2 genes are directly transactivated by p53 in response to stress, whereas Bcl-2 is an anti-apoptotic gene repressed by p53.23, 24
Their expression was determined using western blot analysis (Figure 6b), using protein extracts from U2OS-ctrl cells analyzed in Figure 5B. RT-qPCR analysis was also performed in parallel (Supplementary Figure D). Doxorubicin treatment induced the expression of p21 and HDM2 at the mRNA and protein levels, but had no effect on Bcl-2 expression (Supplementary Figure D and Figure 6b lane 2 compared with 1). Depletion of FLp53 by siTA after doxorubicin treatment strongly inhibited p21 and HDM2 at the mRNA and protein levels, without altering Bcl-2 expression (Supplementary Figure D and Figure 6b lane 5 compared with 2). This confirms that in U2OS-ctrl cells, induction of p21 and HDM2 expression in response to a low dose of doxorubicin is p53-dependent, whereas it suggests that Bcl-2 expression in U2OS-ctrl cells is not p53-dependent. Then, we compared with siNS, the effect of Δ133p53α depletion by si133a or si133b on the expression of p21, HDM2 and Bcl-2, in cells treated with doxorubicin (Supplementary Figure D and Figure 6b lanes 3, 4 compared with 2). Depletion of Δ133p53α significantly increased the expression of p21 mRNA, but did not alter p21 protein expression. Interestingly, Δ133p53α depletion reduced the expression of HDM2 and Bcl-2 at both protein and mRNA levels. This suggests that Δ133p53α can increase Bcl-2 expression and contributes to the induction of HDM2 expression, in response to treatment. Therefore, our results show that Δ133p53α can differentially regulate p21, HDM2 and Bcl-2 expression and that Δ133p53α can directly interact with p53.
Altogether, we show that p53-mediated expression of Δ133p53α in response to a low dose of doxorubicin, leads to the inhibition of p53-mediated apoptosis and G1 cell cycle arrest, without inhibiting p53-mediated G2 cell cycle arrest. Moreover, we show that Δ133p53α can directly interact with p53 and can differentially regulate the expression of p21, HDM2 and Bcl-2 in response to doxorubicin. This indicates that Δ133p53α does not exclusively inhibit p53, but rather modulates p53 activities. Of note, we cannot exclude that it can modulate gene expression independently of p53.
Discussion
We have recently reported that the human p53 gene encodes nine different p53 isoforms, which can modulate p53 target gene expression, in a promoter-dependent manner.6 In this study, we investigated the regulation of p53's internal promoter leading to Δ133p53α expression and the biological activities of endogenous Δ133p53α in response to a low dose of doxorubicin.
We determined that the initiation site of the p53 internal promoter is located within the last 250 bp of intron-4 of the human p53 gene. Moreover, we identified a functional p53RE composed of five putative decamers at the junction of exon-4 and intron-4. Further analysis will determine whether all five decamers can confer responsiveness to p53, p63 and/or p73. By bioinformatics analysis, we determined that the p53 internal promoter could be responsive to cell proliferation signals (AP1, Elk1, N-myc and E2F), hypoxia (HIF-1α), heat shock (HSF1) or hormones (estrogen receptor). In addition, we identified a silencer region (position 1042–1304) containing response elements to the repressor ZNF219 and CBF1.
To date, it is not possible to specifically quantify the expression of each separate Δ133p53 isoform (Δ133p53α, Δ133p53β and Δ133p53γ), encoded from the p53 internal promoter. Hence, to quantify the mRNA level of the Δ133p53 isoforms, we designed primers and probe specific for the 5′UTR of Δ133p53 mRNAs, absent in FLp53 mRNA. We showed that the human p53 internal promoter is induced in response to doxorubicin or Act D treatment in normal and in cancer cell lines expressing WTp53, suggesting that the p53-mediated regulation of its own internal promoter is conserved through evolution.25
Consistently, the expression of Δ133p53 mRNAs is associated with an increase in Δ133p53α protein level only in normal cells and in cancer cell lines expressing WTp53 as determined by transfection of two distinct siRNAs (si133a and si133b), specific for the Δ133p53 mRNA variants. The endogenous Δ133p53α protein corresponds to a band at 35 kDa. It is noteworthy that ectopic and endogenous Δ133p53α give a band at 35 kDa and a weaker band at 32 kDa, in U2OS cells. We assume that the band at 32 kDa corresponds to a post-translationally modified form of Δ133p53α.
We have recently published that Δ113p53, the zebrafish homolog of human Δ133p53α, prevents p53-mediated apoptosis in response to embryonic defects or DNA damage25 and that human Δ133p53α can inhibit replicative senescence.14 We investigated whether the cellular response to DNA damage could be regulated by modulating Δ133p53α expression. We determined that treatment of U2OS-ctrl cells with doxorubicin (0.5 μM, 1 h) induces exclusively p53-dependent G2 cell cycle arrest without promoting G1 cell cycle arrest and p53-dependent apoptosis. Then, we established that depletion of Δ133p53 mRNAs by si133a or si133b changes the cellular response of U2OS cells treated with doxorubicin (0.5 μM, 1 h), increasing the proportion of cells undergoing apoptosis and G1 cell cycle arrest. Overexpression of Δ133p53α restores resistance to apoptosis and abolition of G1 cell cycle arrest without preventing p53-mediated G2 cell cycle arrest. The inhibition of p53-mediated apoptosis and G1 cell cycle arrest in response to Δ133p53α expression is consistent with our previous reports showing that Δ133p53α can function in a dominant-negative manner toward p53.6, 7, 14 The direct interaction of Δ133p53α with p53 is in support of this mechanism. However, as the overexpression of Δ133p53α did not inhibit p53-dependent G2 cell cycle arrest, it indicates that Δ133p53α does not exclusively act by inactivating p53. Indeed, Δ133p53α represses p21, whereas it induces Bcl-2 and HDM2 expression in response to doxorubicin, suggesting that many genes are differentially regulated by Δ133p53α to modulate cell fate outcome in response to DNA damage. Further experiments will determine whether it can regulate gene expression independently of p53. Our findings may have profound significance to extend our understanding of the mechanisms, by which p53 exerts its tumor suppressor activity. They demonstrate a new feedback loop, in which p53-induced Δ133p53α functions to modulate the cellular response to DNA damage.
Materials and Methods
p53 internal promoter constructs
The p53 internal promoter, corresponding to the region 11 523–13 076 bp of the human p53 gene (accession no. X54156, NCBI), was generated by PCR using the primers p53RE-a fwd and p53RE-a rev (Table 1). The PCR fragment was first cloned into the pCR4-TOPO vector using the TOPO TA Cloning kit (Invitrogen, Paisley, UK) and was further subcloned into the pGL3-basic plasmid, upstream of the luciferase reporter gene, to generate the pi3i4-luc construct (construct a in Figure 1A). Subsequently, a number of deletion constructs of pi3i4-luc (b, c, d and e in Figure 1A) were made by enzymatic digestion using various restriction enzymes, followed by religation and subcloning of the obtained fragments. Two other pi3i4-luc constructs (constructs c and d in Figure 2A), with or without p53REs, were generated by PCR using the primers' combination p53RE-b fwd/p53RE-a rev and p53RE-c fwd/p53RE-a rev for constructs b and c, respectively (Figure 2A and Table 1). Site-directed mutagenesis of the identified p53REs was carried out by PCR on the pi3i4-luc, using the primers Mut-p53RE fwd and Mut-p53RE rev (Table 1). All generated constructs were verified by DNA sequencing.
Dual luciferase assay
H1299 cells (3 × 104) were seeded in 24-well plates, 24 h before transfection. Two hours before transfection, media were replaced with 0.5 ml of fresh complete DMEM. Each transfection was performed in duplicate, and all results shown are the average of at least three separate individual experiments. For each well, 0.5 μg of each p53 internal promoter construct (as indicated in the legends) and 50 ng of the Renilla luciferase reporter plasmid were co-transfected with 200 ng of the FLp53 expression vector or with the pSI (empty expression vector), used as negative control. The Renilla luciferase reporter plasmid was used as an internal control. Luciferase assays were performed as previously described.26
Cell culture and generation of stable cell lines
The different human cell lines used in our study were: the non-small lung carcinoma cell line H1229 (p53-null), the osteosarcoma cell line U2OS (WTp53), the colon cell lines HCT116 (WTp53), the breast cancer cell line MCF7 (WTp53) and the primary NHDF. The MCF7 stable line overexpressing a dominant negative form of p53 (MCF7-DDp53)27 was also used. The HCT116−/−, devoid of FLp53 expression, were kindly provided by Professor Vogelstein's lab (The Ludwig Center for Cancer Genetics and Therapeutics, Howard Hughes Medical Institute and Sidney Kimmel Cancer Center at the Johns Hopkins Medical Institutions, Baltimore, MD, USA).17 Two U2OS stable cell lines were generated after transfection with either the empty pcDNA-3 expression vector (U2OS-ctrl) or with the pcDNA3-Δ133p53 expression vector (U2OS-Δ133p53). The heterogeneous populations of both cells lines were used in the experiments, no clones were isolated. NHDF cells were kindly provided by Dr C Pourreyron (University of Dundee, Ninewells Hospital, UK). H1299 cells were cultured in RPMI medium supplemented with 10% fetal calf serum and 100 μg/ml penicillin/streptomycin. All the other cell lines were cultured in DMEM medium supplemented with 10% fetal calf serum and 100 μg/ml penicillin/streptomycin.
RNA extraction, cDNA synthesis, RT-qPCR and ChIP assay
Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Crawley, UK) and treated with DNase (Qiagen) before reverse transcription, which was carried out using random hexamers (Promega, Southampton, UK) and Superscript II reverse transcriptase (Invitrogen). qPCR was performed on Stratagene MX3005P. p53 specific primers and probes were designed in our laboratory (Table 1) and purchased from MWG (Eberberg, Germany). RT-qPCR analyses for the p21, Bax, HDM2 and Bcl2 genes were performed as previously described.28, 29 All measurements were normalized to the expression of the TATA box-binding protein (TBP) gene. p53 specific ChIP assay was conducted on MCF7 cells, as previously described using anti-p53 monoclonal antibody, DO-1.6
Western blot analysis
Cells lysates were separated by 4–12% SDS-PAGE (Novagen system, Birmingham, UK), transferred to a nitrocellulose membrane and blotted with various antibodies. The primary antibodies used were the following: anti-p53 CM1,30 anti-HDM2 4B2,28 p21 (Calbiochem, Nottingham, UK), PARP (Roche, Welwyn Garden City, UK), Bax, Bcl2 and actin (Santa Cruz, Heidelberg, Germany). All secondary horseradish peroxidase conjugated antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Immunoprecipitation assay
H1299 (106 cells per 10-cm dish) were co-transfected with 2 μg pSV-p53 expression vector driven by the SV40 promoter and/or 2 μg pSV-Δ133p53 expression vector driven by the SV40 promoter. For each transfection mix, the concentration of the SV40 promoter was balanced with the SV40 empty expression vector. Fugene (Boehringer, Bracknell, UK) was used as a transfection reagent. Immunoprecipitation was carried out as previously described6 with anti-p53 mouse monoclonal antibody DO-1.
Protein content was then analyzed by western blot with anti-p53 rabbit polyclonal antibody (CM1), as previously described.6
siRNA transfection, apoptosis and cell cycle analyses
U2OS (105 cells per 6-well plate) were transfected with the appropriate siRNA using oligofectamine (Invitrogen), treated with doxorubicin and further incubated for 24 h before harvesting for apoptosis and cell cycle assays. The efficiency of si133a, si133b and siTA was assessed by RT-qPCR (Supplementary Figures C1 and C2). Apoptotic cells were quantified using an Annexin V-fluorescein isothiocyanate kit (Biosciences, Cambridge, UK), according to the manufacturer's instructions and were analyzed by flow cytometry (Becton Dickinson, Oxford, UK). Cell cycle analysis was carried out using BrdU (Sigma, Bookham, UK) pulse labeling and flow cytometry, as previously described31 and according to the manufacturer's instructions.
Accession codes
Abbreviations
- p53RE:
-
p53 responsive element
- siRNA:
-
small interfering RNA
- siNS:
-
non-specific siRNA
- si133a:
-
siRNA specific for Δ133p53 mRNAs
- si133b:
-
siRNA specific for Δ133p53 mRNAs
- siTA:
-
siRNA targeting specifically exon-2 of p53 mRNAs
References
Royds JA, Iacopetta B . p53 and disease: when the guardian angel fails. Cell Death Differ 2006; 13: 1017–1026.
Braithwaite AW, Prives CL . p53: more research and more questions. Cell Death Differ 2006; 13: 877–880.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Lavin MF, Gueven N . The complexity of p53 stabilization and activation. Cell Death Differ 2006; 13: 941–950.
Laptenko O, Prives C . Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ 2006; 13: 951–961.
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev 2005; 19: 2122–2137.
Murray-Zmijewski F, Lane DP, Bourdon JC . p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 2006; 13: 962–972.
Boldrup L, Bourdon JC, Coates PJ, Sjostrom B, Nylander K . Expression of p53 isoforms in squamous cell carcinoma of the head and neck. Eur J Cancer 2007; 43: 617–623.
Ebrahimi M, Boldrup L, Coates PJ, Wahlin YB, Bourdon JC, Nylander K . Expression of novel p53 isoforms in oral lichen planus. Oral Oncol 2008; 44: 156–161.
Bourdon JC . p53 and its isoforms in cancer. Br J Cancer 2007; 97: 277–282.
Anensen N, Oyan AM, Bourdon JC, Kalland KH, Bruserud O, Gjertsen BT . A distinct p53 protein isoform signature reflects the onset of induction chemotherapy for acute myeloid leukemia. Clin Cancer Res 2006; 12: 3985–3992.
Avery-Kiejda KA, Zhang XD, Adams LJ, Scott RJ, Vojtesek B, Lane DP et al. Small molecular weight variants of p53 are expressed in human melanoma cells and are induced by the DNA-damaging agent cisplatin. Clin Cancer Res 2008; 14: 1659–1668.
Marabese M, Marchini S, Marrazzo E, Mariani P, Cattaneo D, Fossati R et al. Expression levels of p53 and p73 isoforms in stage I and stage III ovarian cancer. Eur J Cancer 2008; 44: 131–141.
Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol 2009; 11: 1135–1142.
Moll UM, Slade N . p63 and p73: roles in development and tumor formation. Mol Cancer Res 2004; 2: 371–386.
Bourdon JC, Deguin-Chambon V, Lelong JC, Dessen P, May P, Debuire B et al. Further characterisation of the p53 responsive element – identification of new candidate genes for trans-activation by p53. Oncogene 1997; 14: 85–94.
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998; 282: 1497–1501.
Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 2002; 21: 6722–6728.
Allan LA, Fried M . p53-dependent apoptosis or growth arrest induced by different forms of radiation in U2OS cells: p21WAF1/CIP1 repression in UV induced apoptosis. Oncogene 1999; 18: 5403–5412.
Medema RH, Klompmaker R, Smits VA, Rijksen G . p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA-replication or stress-activated kinases. Oncogene 1998; 16: 431–441.
Juven T, Barak Y, Zauberman A, George DL, Oren M . Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene 1993; 8: 3411–3416.
Barak Y, Gottlieb E, Juven-Gershon T, Oren M . Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev 1994; 8: 1739–1749.
Miyashita T, Harigai M, Hanada M, Reed JC . Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res 1994; 54: 3131–3135.
Bredow S, Juri DE, Cardon K, Tesfaigzi Y . Identification of a novel Bcl-2 promoter region that counteracts in a p53-dependent manner the inhibitory P2 region. Gene 2007; 404: 110–116.
Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP et al. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev 2009; 23: 278–290.
Bourdon JC, Renzing J, Robertson PL, Fernandes KN, Lane DP . Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J Cell Biol 2002; 158: 235–246.
Shaulian E, Haviv I, Shaul Y, Oren M . Transcriptional repression by the C-terminal domain of p53. Oncogene 1995; 10: 671–680.
Saville MK, Sparks A, Xirodimas DP, Wardrop J, Stevenson LF, Bourdon JC et al. Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J Biol Chem 2004; 279: 42169–42181.
Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK . The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J 2007; 26: 976–986.
Midgley CA, Fisher CJ, Bartek J, Vojtesek B, Lane D, Barnes DM . Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J Cell Sci 1992; 101 (Pt 1): 183–189.
Hoy CA, Carswell C, Schimke RT . Bromodeoxyuridine/DNA analysis of replication in CHO cells after exposure to UV light. Mutat Res 1993; 290: 217–230.
Acknowledgements
Mustapha Aoubala is supported by Breast Cancer Campaign, Fiona Murray-Zmijewski was supported by Breast Cancer Campaign and MRC. Marie P Khoury and Stephane Perrier are supported by Inserm (Institut National de la Santé Et de la Recherche Médicale), Hugo Bernard is supported by Ligue Nationale Contre le Cancer. Kenneth Fernandes and Jean-Christophe Bourdon are supported by Cancer-Research, UK. We thank Drs Mark Saville and Alice Marchado-Silva for their constructive discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Edited by M Oren
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Aoubala, M., Murray-Zmijewski, F., Khoury, M. et al. p53 directly transactivates Δ133p53α, regulating cell fate outcome in response to DNA damage. Cell Death Differ 18, 248–258 (2011). https://doi.org/10.1038/cdd.2010.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2010.91
Keywords
This article is cited by
-
Cyclin-dependent kinase 7 (CDK7) inhibitors as a novel therapeutic strategy for different molecular types of breast cancer
British Journal of Cancer (2024)
-
Elevation of effective p53 expression sensitizes wild-type p53 breast cancer cells to CDK7 inhibitor THZ1
Cell Communication and Signaling (2022)
-
Synthetic lethality between TP53 and ENDOD1
Nature Communications (2022)
-
Δ133p53β isoform pro-invasive activity is regulated through an aggregation-dependent mechanism in cancer cells
Nature Communications (2021)
-
Functional interplay between p53 and Δ133p53 in adaptive stress response
Cell Death & Differentiation (2020)