Abstract
CD95 is a dual-function receptor that exerts pro- or antiapoptotic effects depending on the cellular context, the state of activation, the signal threshold and the mode of ligation. In this study, we report that CD95 engagement modulates TCR/CD3-driven signaling pathways in resting T lymphocytes in a dose-dependent manner. While high doses of immobilized CD95 agonists silence T cells, lower concentrations augment activation and proliferation. We analyzed the co-stimulatory capacity of CD95 in detail in resting human CD4+ T cells, and demonstrate that low-dose ligand-induced co-internalization of CD95 and TCR/CD3 complexes enables non-apoptotic caspase activation, the prolonged activation of MAP kinases, the upregulation of antiapoptotic proteins associated with apoptosis resistance, and the activation of transcription factors and cell-cycle regulators for the induction of proliferation and cytokine production. We propose that the levels of CD95L on antigen-presenting cells (APCs), neighboring T cells or epithelial cells regulate inhibitory or co-stimulatory CD95 signaling, which in turn is crucial for fine-tuning of primary T-cell activation.
Similar content being viewed by others
Main
For activation of resting T cells, two signals are required. The first signal emerges from an engagement of the T cell receptor (TCR)/CD3 complex, whereas the second signal is generated through the ligation of co-stimulatory receptors (i.e. CD28). Recently, ‘tumor necrosis factor (TNF) receptor-associated factor’ (TRAF) binding receptors were identified as a second class of co-stimulatory receptors.1 As an example, Alderson et al.2, 3 provided first evidence for the role of CD95 (Fas, APO-1), the prototypic death receptor of the immune system, in the activation of human T cells. It was subsequently reported that CD3-crosslinking alone or CD3/CD95 co-stimulation induces the processing of caspase-8 and/or caspase-3 as a prerequisite for full T-cell proliferation.4, 5, 6
CD95 co-ligation also influences several other routes of intracellular signal transduction. Kataoka et al.7 reported the activation of NF-κB- and mitogen-activated protein kinase (MAPK)-related pathways following an interaction of CD95-recruited ‘cellular FLICE-inhibitory protein’ (cFLIP) with downstream signaling molecules. Apparently, this process required the cleavage of cFLIP into a p43 fragment. More recently, however, it was argued that p22-FLIP (but not p43) can activate NF-κB by directly interacting with the IKK complex.8 So far, three cFLIP isoforms (cFLIPL, cFLIPS and cFLIPR) were identified, with cFLIPS/R mediating a block in apoptosis by inhibiting procaspase-8 at the death-inducing signaling complex (DISC). The role of cFLIPL regarding an inhibition at the DISC is still a matter of debate.8, 9 Further downstream, antiapoptotic proteins including Bcl-2/Bcl-XL and ‘X-linked inhibitor-of-apoptosis protein’ (XIAP) may prevent apoptosis.9, 10 A decreased expression of antiapoptotic checkpoint proteins in CD95-sensitive cells could therefore shift NF-κB-associated proliferative signaling pathways to caspase-associated death signaling in the course of cellular activation.11
We now report that CD95 engagement modulates the activation of primary human CD4+ T cells in a dose-dependent manner. Whereas high concentrations of CD95 agonists result in the block of activation, low concentrations augment TCR-induced proliferation. As this differential signaling capacity was seen for different ligands, it points to a novel mechanism to regulate T-cell activation in a context-specific manner. In addition to the inhibitory effect (recently described by Strauss et al.12) we provide a detailed analysis of signaling events associated with the positive co-stimulatory function of CD95. We demonstrate that CD95 ligation with low doses of agonists promotes TCR-triggered MAPK phosphorylation, non-apoptotic caspase activation, and the upregulation of activation markers and antiapoptotic checkpoint proteins. As a consequence, enhanced cell-cycle progression, proliferation and cytokine production are associated with a generalized partial apoptosis resistance.
Results
Modulation of primary T-cell activation by CD95 engagement
To analyze the effects of CD95 ligation on the TCR/CD3/CD28-induced activation of freshly isolated peripheral blood mononuclear cells (PBMCs), we initially immobilized anti-CD3 monoclonal antibody (mAb) +/− anti-CD28 mAb in the presence or absence of CD95LFc, huIgGFc or agonistic anti-CD95 mAb (7C11). As shown in Figure 1a, TCR-induced proliferation was significantly reduced in the presence of CD95LFc, but augmented in the presence of anti-CD95 mAb. Immobilization was mandatory to modulate T-cell activation, as soluble factors had no effect (not shown). Moreover, stimulation by co-immobilized anti-CD28 mAb did not alter the outcome. When we analyzed purified CD4+ T cells (Figure 1b–d), we observed an almost complete block of TCR-induced cluster or blast formation and proliferation in the presence of CD95LFc, but a massive activation using anti-CD95 mAb (anti-APO-1). The CFSE profiles indicated that upon CD3/CD28 stimulation, anti-CD95 co-ligation induced cell-cycle progression in the entire population, whereas in the absence of anti-CD95 significantly fewer cells divided. Importantly, neither immobilized CD95LFc nor immobilized anti-CD95 mAb induced significant cell death of resting T cells during the observation period (Figure 1e).
CD95 stimulation affects activation of primary T cells without induction of cell death. (a) Freshly isolated human PBMCs were stimulated for 3 days on 96-well plates coated with anti-CD3 or anti-CD3/anti-CD28 in the presence or absence of anti-CD95 mAb (here 7C11, 2 μg/ml), 20 μg/ml CD95LFc or huIgGFc as a control. Proliferation was determined by adding [3H] TdR for 16 h before harvesting. This experiment was performed in triplicates. Error bars indicate the S.D. of the mean values. (b–e) Purified CD4+ T cells were cultured in X-VIVO medium for 3 days in 24-well plates coated with anti-CD3/anti-CD28 in the presence or absence of anti-CD95 mAb (here anti-APO-1, 5 μg/ml), CD95LFc or Fc control protein (both 20 μg/ml). (b) Microphotographs document the increase in cell size and cluster formation as observed by inverse light microscopy. (c) Blastogenesis on the basis of cell size (forward scatter) and granularity (side scatter) was determined by FACS analysis. (d) Freshly isolated human CD4+ T cells were labeled with CFSE and stimulated as indicated. CFSE profiles were analyzed by flow cytometry. (e) Cell death of stimulated CD4+ T cells was investigated by PI-staining and FACS analysis. All experiments were performed with freshly isolated cells from different donors, and are representative of at least three independent experiments performed
Dose-dependent effects of CD95 co-ligation
As the effects of immobilized CD95 agonists were seen at coating concentrations of 20–40 μg/ml for fusion proteins and 2–5 μg/ml for mAb, we wondered whether the co-stimulatory function would depend on the dose of agonists. To test this, CD4+ T cells were stimulated with anti-CD3/CD28 in the absence or presence of CD95 agonists that were co-immobilized at various concentrations. T-cell activation was monitored by the expression of CD25 and CD69 at d1 (Figure 2). Although CD95LFc, CD95L-ST-Fc, LZ-CD95L and anti-APO-1 at high concentrations inhibited the expression of both activation markers, lower amounts of agonists led to an increased number of CD25/CD69 double-positive cells. This dose-dependency was seen for all ligands tested, although the degree of inhibition and stimulation varied to some extent between individual donors. Of note, the apoptosis-inducing capacity of all CD95 reagents was confirmed using pre-activated phytohemagglutinin (PHA) blasts and soluble agonists (Supplementary Figure S1). In addition, as exemplified for LZ-CD95L, at the tested concentrations, soluble CD95 ligands alone only mildly affect the viability of the freshly isolated CD4+ T-cell population.
CD95 ligands affect T-cell activation in a dose-dependent manner. MACS-purified human CD4+ T cells from three different donors were cultured overnight in X-VIVO medium in 96-well plates coated with or without anti-CD3 mAb and anti-CD28 mAb in the presence or absence of graded doses of plate-bound CD95LFc, CD95L-ST-Fc, LZ-CD95L or anti-CD95 mAb anti-APO-1 as indicated. The expression levels of CD25 and CD69 were monitored by flow cytometry. The percentage of CD25/CD69 double-positive cells is indicated in individual dot plots
CD95 co-stimulation augments TCR-induced cytokine production
Co-immobilization of anti-CD95 augmented the production of IL-2, interferon gamma (IFNγ) and TNFα, but hardly influenced the level of IL-4 (Figure 3). As expected, IL-2 production depended on CD3 stimulation and was further enhanced by CD28 co-stimulation (Figure 3a). CD95 ligation alone had no effect. In the presence of anti-CD95, CD3-stimulated T cells initially produced less IL-2 compared to CD3/CD28-triggered cells. However, higher levels of IL-2 were detected at d2/3 by ELISA, and also by intracellular FACS staining (Supplementary Figure S2A). To document the strong co-stimulatory capacity of CD95 irrespective of the used agonist, the data were verified by co-stimulation with low amounts of CD95LFc (Supplementary Figure S2B). In line with the growth inhibition at high doses of CD95LFc, also the IL-2 production was reduced compared to CD3-stimulated cells (Supplementary Figure S2B). The presence of exogenous IL-2 hardly affected the activation-induced CD25 expression (Figure 3b and Supplementary Figure S2C), arguing that the CD3/28/95-induced IL-2 production sufficed for optimal initiation of T-cell activation. Also, the CD3/CD28-induced production of IFNγ and TNFα was significantly enhanced in the presence of low doses of anti-CD95 (Figure 3c). As shown in Supplementary Figure S3A, similar results were obtained using low versus high doses of other agonists, for example, CD95L-ST-Fc. Notably, IL-4 production was almost unchanged, indicating a more pronounced effect of CD95 ligation on T helper 1 (Th1)-type cells. Consistent with this, T-bet, a known regulator of Th1 differentiation, was upregulated and phosphorylation of STAT-1 and STAT-4 was enhanced only in the presence of low-dose anti-CD95 (Figure 3d) or CD95L-ST-Fc (Supplementary Figure S3B).
The low-dose co-stimulatory effect of CD95 is associated with IL-2 production and potentially skews a Th1 response. Freshly isolated CD4+ T cells were left untreated or treated with immobilized anti-CD3 mAb plus/minus anti-CD28 mAb in the absence or presence of 5 μg/ml anti-APO-1. (a) Secreted IL-2 was determined in supernatants by ELISA after 24, 48 and 72 h. Experiments were performed in triplicates with error bars indicating S.Ds., and are representative for three experiments carried out with different donors. (b) Exogenous rIL-2 was added where indicated. At day 3 of culture, CD25 expression was analyzed by flow cytometry using a PE-labeled anti-CD25 mAb. The percentages of CD25-positive cells are indicated in individual dot plots. (c) IFNγ, IL-4 and TNFα were measured by intracellular staining at d3. The experiment was performed in triplicates. Cytokine production is given as mean fluorescence intensities (MFI) for one representative experiment out of five with different donors. Error bars indicate S.Ds. from the MFI. Statistical differences were calculated by a standard t-test: *P<0.05; **P<0.01; ***P<0.001. (d) The expression level of T-bet and the phosphorylation of STAT1 and STAT4 were determined in cell lysates of primary human CD4+ T cells after 3 days of stimulation. Western Blot analysis was performed as described using specific antibodies against T-bet, p-STAT1, p-STAT4 and ERK as a loading control
CD95 affects the expression of activation markers and TCR-associated signaling pathways
As shown in Figure 4 and Supplementary Figure S4, starting at 2–4 h of incubation, CD69 expression on CD3-, CD3/CD28- or PHA-stimulated CD4+ T cells was higher in the presence of low doses of anti-CD95 or CD95L-ST-Fc. A sustained high level of CD69 following co-stimulation with low dose of anti-CD95 (Figure 4b) or CD95L-ST-Fc (Supplementary Figures S5, S6 and S7) was detected at d2/3, along with massive increases of other activation markers including OX-40 (CD134), IL-2Rα (CD25), IL-2Rβ (CD122), cytotoxic T-lymphocyte antigen-4 (CTLA-4) (CD152) and CD95L (CD178). In contrast, high doses of CD95L-ST-Fc completely blocked activation (Supplementary Figures S6 and S7).
CD95 promotes upregulation of activation markers and ERK activation. Purified human CD4+ T cells were incubated in X-VIVO medium with immobilized anti-CD3 mAb or PHA for the indicated time periods in the presence or absence of plate-bound anti-CD95 mAb anti-APO-1 (5 μg/ml) (a–c, e and f) or CD95L-ST-Fc (2,5 μg/ml) (e) as well as high doses of CD95L-ST-Fc (d) or CD95LFc (f) (both 20 μg/ml). The expression of CD69 (a and b) and other activation markers (b) was determined between 1–8 h (a), and at d2 (b) of incubation by flow cytometry using FITC/PE-labeled anti-CD69, anti-OX40, anti-CTLA-4, anti-CD25, anti-IL-2Rβ and anti-CD95L mAb, respectively. Expression levels are given as MFI and are representative of three independent experiments with different donors. (c–d) After incubation at 37°C for the indicated time points, T cells were lysed and aliquots of 20 μg of the whole-cell lysate were analyzed by western blot using antibodies against phospho-ERK and ERK as a loading control. (e) Cells were treated in the presence or absence of the ERK1/2 inhibitor PD 98059 (50 μM) or DMSO for 3 days before MTS assay. The experiment was performed in triplicates. Error bars represent S.Ds. from the mean. Statistical differences were calculated by student's t-test: **P<0.01. (f) CFSE-labeled CD4+ T cells were incubated in the presence of PMA, ionomycin or PMA/ionomycin with or without 20 μg/ml CD95LFc or 2 μg/ml anti-CD95 mAb (7C11), respectively. CFSE profiles were measured at d3
When comparing CD3-stimulated and CD3/CD95-stimulated cells regarding the kinetics of extracellular signal-regulated protein kinase (ERK) phosphorylation, we did not observe major differences in short-term cultures up to 30 min. However, whereas ERK phosphorylation of TCR/CD3-triggered cells was transient and declined thereafter, in the presence of anti-CD95, we detected a prolonged phosphorylation for up to 48 h (Figure 4c). This enhanced ERK activation was also seen for low-dose CD95L-ST-Fc (Supplementary Figure S8). In this scenario, treatment with the ERK1/2 inhibitor PD 98059 (PD) significantly blocked cell activation and proliferation (Figure 4e, Supplementary Figure S9) in the absence of cell death (Supplementary Figure S9A), indicating that ERK signal transduction is crucial for the antiapoptotic CD95-mediated co-stimulatory capacity. In contrast, and in line with the report by Strauss et al.,12 T cells co-incubated with high concentrations of CD95 agonists showed a drastically reduced ERK phosphorylation already within the first minutes (Figure 4d) and still at d2 of incubation (Supplementary Figure S8A). Notably, T-cell activation induced by phorbolester (PMA) and calcium ionophore (ionomycin) was not affected by CD95 ligation, neither at high nor at low concentrations (Figure 4f).
CD95 crosslinking enhances TCR internalization
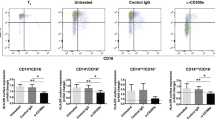
TCR/CD3 complex internalization is associated with optimal activation through the formation of intracellular signaling platforms.13 As shown in Figure 5a and b and Supplementary Figure S10A,B, FACS analyses revealed a significant augmentation of CD3-driven TCR internalization by low-dose CD95 co-stimulation within the first 8 h of incubation, and even more pronounced after 48 h. At the same time, CD69 expression was upregulated, indicating that CD95 crosslinking selectively down-modulated TCR surface availability (Supplementary Figure S10B).
TCR and CD95 internalization are enhanced in TCR-triggered cells co-treated through low-dose CD95. Freshly isolated CD4+ T cells were incubated with plate-bound anti-CD3/CD28 mAb (a and b) or PHA (c–e) in the presence or absence of immobilized anti-APO-1 (5 μg/ml). (a and b) TCR internalization was analyzed using a FITC-labeled TCRα/β-specific mAb between 2 and 8 h (a) and after 48 h of stimulation (b). The experiments were performed in triplicates. Error bars represent S.Ds. from the mean. Statistical differences were calculated by student's t-test: *P<0.05. Dead cells were excluded by FSC/SSC gating. (c–e) After stimulation for 30 min to 48 h as indicated, cells were harvested and incubated on ice with 1 μg anti-APO-1 per 106 cells and PE-labeled goat-anti-mouse mAb. CytD or LatA was added where indicated. (c) Expression levels of CD95 from four different donors are denoted in MFI expressed on a logarithmic scale. The crossbar represents the arithmetic mean. Statistical significance was determined using student's t-test: *P<0.05. (d and e) Data are depicted as MFI for one representative experiment out of three with T cells from three different donors
CD95 triggering is associated with receptor internalization
Also for members of the TNF receptor (TNFR) superfamily, receptor internalization has been associated with the generation of intracellular signaling platforms. In case of TNFR1 or CD95, internalized receptosomes contain several pro-apoptotic molecules.14 To test whether CD95 internalization might also be associated with non-apoptotic CD95 signaling, we analyzed CD95 surface expression following stimulation (Figure 5c–e, Supplementary Figure S10C). In the absence of CD3 stimulation, low-dose CD95 ligation reduced CD95 surface availability (without affecting T-cell activation). Although TCR or mitogen activation of resting T cells was associated with an upregulation of CD95, this increased expression was significantly counter-regulated in the presence of anti-CD95 (Supplementary Figure S10C). Importantly, CD69 expression followed the patterns described before (Figure 5d). Using a set of inhibitors for metalloproteases (GM6001, TAPI), clathrin-dependent endocytosis (monodansyl cadaverine), microtubule (colchicine) and actin polymerization (cytochalasin D (CytD), latrunculin A (LatA)), we observed a dose-dependent reduction of CD95 internalization only in the presence of CytD and LatA (Figure 5e), indicating that an actin-dependent mechanism might control this process. Because of the inhibition of actin polymerization, we observed a complete block of CD3/CD28-driven T-cell proliferation (Supplementary Figure S10D) without detectable cell death (Supplementary Figure S10E). Thus, we suggest that abolished CD95 internalization results in T-cell silencing.
CD95 co-stimulation induces caspase activity and expression of antiapoptotic proteins
To address whether CD95-triggered caspase activity would also contribute to TCR-induced activation, we analyzed caspase processing and activity (Figure 6). In line with earlier reports,4, 5, 6, 10, 15 increased caspase-3/-8 activities were detected in lysates of TCR-stimulated cells, and caspase activity was further enhanced by co-stimulation with anti-CD28 or low amounts of anti-CD95 or CD95LFc. The importance of active caspases in this scenario was illustrated by the addition of the pan-caspase inhibitor ZVAD-fmk. Caspase inhibition resulted in a drastic reduction of CD25 expression and inhibited proliferation without provoking apoptosis (Supplementary Figure S11). Interestingly, caspase activation during positive CD95 co-stimulation was even higher than the activity seen in apoptotic Jurkat T cells (Figure 6a, lower panels). In contrast, CD95 ligation using CD95LFc at high concentrations completely blocked caspase activation in resting cells. Positive CD95 co-stimulation significantly increased the level of cleaved caspase-3 (p20) (Figure 6b and c). As shown in Figure 6c, the partial caspase-3 processing was associated with an increase of active caspase-8 (p41/p43). Although the p20 product of caspase-3 and the p41/p43 intermediates of caspase-8 seem to be capable of cleaving the fluorogenic caspase-3/-8 substrates, we did not observe a significant cleavage of apoptosis-associated substrates (i.e., PARP, phospholipase Cγ (PLCγ). Therefore, pro- and antiapoptotic caspase signaling might depend on a differential processing of caspase-3. Taken together, we show that activation of primary T cells correlates with caspase processing and in vitro caspase activity.
Low-dose CD95 co-engagement induces caspase activation, expression of antiapoptotic proteins and promotes apoptosis resistance. Caspase-3/-8 activity (a) and processing (b and c) as well as cleavage of caspase substrates (c) were determined after incubation of primary human TCR-stimulated cells in the presence or absence of the indicated CD95 agonists at different concentrations. (a) Cleavage of the caspase-3/-8 specific substrates Ac-DEVD-AMC/Ac-IETD-AMC, followed by the release of the fluorogenic AMC, was used to detect activity of caspase-3/-8 in lysates from T cells incubated for 3 days with or without anti-CD3 +/− anti-CD28 mAb in the presence or absence of anti-CD95 mAb (anti-APO-1, 5 μg/ml (C3)) or CD95LFc (2.5 (C2) and 40 μg/ml (C1)). An AMC fluorescence reference standard was used to calibrate the AMC-based caspase substrates in order to quantify caspase activities (a, lower panel). In this study, we included the caspase activity of Jurkat T cells incubated with 5 μg/ml of soluble CD95LFc for 2 h to induce apoptosis. (b) Resting T cells were stimulated as indicated in the presence or absence of anti-APO-1 (5 μg/ml). Cleavage of caspase-3 was analyzed by western blot at day 1–3. (c) After 2 days of stimulation, T-cell lysates were analyzed by western blot for processed caspase-3 and -8, and the cleavage of PLCγ and PARP. ERK was used as a loading control. (d) Similarly, levels of cFLIP, Bcl-XL, IκB and phospho-IκB were determined by western blot. (e) To determine apoptosis resistance, CD4+ T cells were incubated with immobilized anti-CD3 alone or in combination with anti-APO-1 or left untreated. At d2, the cells were collected, washed and exposed to γ-irradiation or mistletoe lectin and incubated overnight. Untreated control cells were left on coated plates used for the initial stimulation. Induction of cell death was determined by PI-staining
Apoptotic death receptor signaling can be intrinsically regulated at several levels by cFLIPR/S, p43- and p22-FLIP as well as antiapoptotic Bcl-2 family members like Bcl-XL. As illustrated in Figure 6d, TCR stimulation resulted in a mild upregulation of Bcl-XL, cFLIPR/S and p22-FLIP. Low-dose anti-APO-1 co-ligation strongly enhanced the expression of all antiapoptotic proteins that were tested (Figure 6d). As NF-κB activation is linked to cFLIP signaling, we also observed enhanced phosphorylation of IκB. Upregulation of cFLIPR/S, Bcl-XL and activation of NF-κB are associated with cell survival. Therefore, we asked whether CD95 co-stimulated cells also display apoptosis resistance to other stimuli. Upon TCR stimulation and more pronounced upon co-stimulation by CD95, we indeed observed a partial apoptosis resistance when the pretreated cells were collected at d2, and either γ-irradiated or exposed to toxic mistletoe lectin16 (Figure 6e).
Accelerated cell-cycle progression of TCR-stimulated cells by low-dose CD95 co-ligation
We next addressed whether CD95 co-stimulation influences cell-cycle progression during primary T-cell activation (Figure 7). Although anti-CD95 alone did not influence the cell-cycle profile, CD3 and CD3/28 stimulation resulted in the expected mild increase of cells within the S- or G2/M-phase. Co-stimulation by anti-CD95, however, resulted in a massive induction of proliferation with 45% of the cells entering S- and G2/M-phase in the absence, and 55% in the presence of anti-CD28. In this study, the co-stimulatory capacity of anti-CD95 was even stronger than the anti-CD28-induced co-stimulation. The differential effect of low versus high dose of agonists on cell-cycle progression was confirmed using the CD95L-ST-Fc fusion protein (Supplementary Figure S12). Similarly, when the T cells were analyzed for the production of ATP, low amounts of CD95L-ST-Fc elevated ATP levels, while high ligand concentrations reduced the anti-CD3/CD28-induced ATP production (Supplementary Figure S12D). At the level of protein expression, we analyzed the appearance of cell-cycle-regulating proteins at d2. Compared to the unchanged level of ERK, all tested cell-cycle-regulating proteins, including CDKs, cyclins and proliferating cell nuclear antigen (PCNA) (see also Supplementary Figure S8A for low versus high dose of CD95L-ST-Fc) and phosphorylated retinoblastoma (Rb) protein, were induced to much higher levels in anti-CD95 co-stimulated cells (Figure 7).
CD95 co-stimulation induces upregulation of cell cycle-regulating proteins and promotes cell-cycle progression. Purified human CD4+ T cells were stimulated with plate-bound anti-CD3 mAb alone or in combination with anti-CD28 mAb. Anti-APO-1 (5 μg/ml) was co-immobilized where indicated. (a) At d3 of incubation, cell-cycle analysis was performed by PI-staining and determination of the DNA content by flow cytometry. Individual phases of the cell cycle, including hypodiploid apoptotic nuclei are indicated. Values denote the percentage of cells in each region. (b) At d3 of culture, the cells were prepared for western blot and analyzed for the expression or phosphorylation of several cell-cycle-regulating proteins using antibodies specific for phospho-Rb (Ser795), phospho-Rb (Ser780), phospho-Rb (Ser807/811), CDKs 1, 2 and 6, cyclins D1, D2, E and B1, PCNA and ERK as a loading control
Discussion
CD95 was originally identified as the prototypic death receptor in and outside the immune system. More recently, it emerged as a dual-function signaling receptor that can give rise to pro- and antiapoptotic signals depending on the cellular microenvironment.2 We now show that CD95 also functions as a dual-function modulator during TCR/CD3-initiated activation of primary human T cells. The outcome of CD95 ligation on naïve T cells largely depends on the ‘dose of agonist’, resulting in opposite effects from a complete block of activation at high doses to a prominent co-stimulatory activation at lower concentrations. In our report, we focus on the growth-promoting function of CD95 on human CD4+ T cells. In essence, CD95 co-stimulation is only seen in the context of TCR/CD3 stimulation, does not require an additional signal (e.g. through CD28), and strictly depends on immobilization of the CD95 agonists.
Antiapoptotic or co-stimulatory consequences of CD95 engagement have been described for different cell types during liver regeneration, neurite outgrowth, development and functional recovery of the central nervous system, and proliferation of growth factor deprived fibroblasts.17 Also, for T lymphocytes, earlier work pointed to a potential modulation of T-cell activation by CD95.3, 4 However, the molecular mechanisms of this co-stimulatory function have never been elucidated. Recently, Strauss et al.12, 18 reported that CD95 co-stimulation blocks the activation of naïve T cells by inhibiting TCR signaling. This is in line with our observations using high concentrations of CD95 agonists, and thus reflects the inhibitory branch of CD95 signaling. As the effective concentrations of antigen-presenting cell (APC)-expressed or plate-bound agonists in the two studies are difficult to correlate, we suggest a narrow threshold window for switching the response from full activation to silencing (supported by our titration experiments). Notably, as for most analyses employing primary human T cells, we also observed donor-related variations of the fraction of co-stimulated cells and the signal amplitude.
A dose-dependency of CD95 ligation was noted earlier for CD95-sensitive SKW6.4 cells.19 Also in this study, CD95 stimulation below a certain threshold level triggered survival, whereas strong CD95 stimulation initiated cell death. However, in contrast to SKW6.4 cells or activated T cells, freshly isolated T cells display apoptosis resistance even to high concentrations of CD95 agonists. As a consequence, high concentrations rather interfere with TCR signal initiation, and ultimately result in T-cell silencing without inducing cell death. In contrast, low concentrations promote a sustained amplification of various TCR-induced activation parameters, including receptor internalization, ERK phosphorylation, expression of antiapoptotic proteins or activation markers, and cell-cycle progression.
For both TCR and CD95 signal initiation, receptor endocytosis has been implicated in the formation of respective signaling platforms important for cellular activation.14, 20 Although the exact mechanisms of TCR internalization are still poorly understood, it in part depends on clathrin-mediated endocytosis.13 Our observations suggest that low-dose CD95 engagement enhances the TCR translocation. However, it is still unclear how this mechanistically relates to CD95 endocytosis and to the generation of a common growth-promoting signaling platform. Notably, an ezrin-dependent route of internalization was recently described to counteract cell death induction and promote antiapoptotic functions of CD95.21 Interestingly in this context, in contrast to CD95-mediated cell death induction, CD95 localization in raft nanodomains has very recently been shown to be dispensable for non-apoptotic CD95 internalization and signaling.22 Our own analyses suggest an accelerated actin-dependent CD95/TCR co-internalization as a mechanism to establish receptor interference and signaling crosstalk.
MAPKs such as ERK are regarded as key switches of cellular activation and proliferation. In the context of low-dose co-stimulatory CD95 signaling, we observed a prolonged ERK phosphorylation. ERK activation in response to non-apoptotic CD95 triggering was reported before for glioma cells23 and fibroblasts.11 Moreover, in primary murine T cells, stimulation with anti-CD3/CD95 caused an ERK-dependent induction of AP-1-dependent luciferase activity.24 Notably, TCR silencing in the presence of high amounts of CD95L is associated with a complete inhibition of CD3/CD28-mediated ERK activation.12 Circumventing TCR ligation with phorbolester and calcium ionophore indicates that CD95 ligation affects TCR signaling upstream or independent of calcium mobilization and PKC activation.
Although caspase activation is a hallmark of apoptosis,9 it has also been associated with lymphocyte activation.4, 15, 25 We indeed detected elevated caspase-3/-8 activities in lysates of proliferating T cells. Co-ligation of CD28 or CD95 led to a comparable increase in caspase activity in this in vitro assay, again indicating that low-dose CD95 co-stimulation might in fact replace CD28 signaling. In agreement with Strauss et al.,12 CD95 agonists at high concentrations blocked caspase activity in the same assay. As pro-apoptotic caspase substrates were never cleaved, we suggest that caspase activation is required for proliferation, but that non- or pro- apoptotic caspase activation is qualitatively different.15 In the case of the effector caspase-3, we only observed the formation of the p20 cleavage fragment, but not of p17/p19, which is found in apoptotic cells. In view of our recent observation that XIAP can interact with caspase-3 and thereby blocks full caspase activation,10 we suggest that in naïve T cells, CD95 initiates an incomplete cleavage of caspase-3, thereby presumably hindering the cleavage of pro-apoptotic substrates. Thus, mitogenic caspase-3 activation might affect a different subset of substrates, which in turn are crucial for mounting a proliferative response. Nonetheless, it is still a matter of debate whether CD95 directly activates caspases via its adapter molecule FADD or whether CD95 only supports the TCR-mediated activation of caspases mediated by a signaling complex formed by Bcl-10, CARMA1 and MALT1.26
Given that cFLIPR/S and the Bcl-2 family member Bcl-XL are known for their capacity to protect from apoptotic cell death,9 we observed a more generalized apoptosis resistance after TCR activation and co-stimulation by CD95. The crucial role of antiapoptotic proteins was underscored by the fact that ABT737, an inhibitor of antiapoptotic Bcl-2 family members, abolished CD3/CD95-mediated apoptosis resistance (not shown). In this context, it has also been noted that antiapoptotic FLIP isoforms do not just block the initiation of the extrinsic apoptotic pathway, but also result in increased survival after TCR engagement and protect from spontaneous apoptosis.27
It was proposed that the expression of CD95L on APC would be responsible for antigen-specific deletion of primed peripheral T cells, eventually leading to T-cell tolerance or immunosuppression.28, 29 More recent data, however, provide evidence for alternate activities of APC-associated CD95L on naïve T cells, including the complete block of T-cell activation in the absence of apoptosis and proliferation.12, 28 This is in line with an earlier report by Chen et al.,30 who observed an inhibition of T-cell proliferation by ‘CD95L-painted’ K562/B7-1 cells that was only partially caused by apoptosis induction. However, in this study, a positive co-stimulatory effect of CD95L had not been noted.
On B cells31 and APC,32 CD95L might be upregulated in the course of an immune response by yet unknown mechanisms accompanying antigen processing and presentation.32 It was suggested that macrophages upregulate CD95L during HIV infection33 and thereby contribute to the apoptotic depletion of uninfected CD4+ T cells. According to the report by Strauss et al.,12 another mechanism of reducing T-cell numbers might be the proliferation block of resting cells. Apparently, the outcome of CD95 ligation very much depends on the state of activation of the respective T-cell population. Also, CD95L-expressing dendritic cells (DCs) trigger apoptosis of pre-activated cells, but induce resistance or even proliferation of naïve T cells.28, 34 Under pathophysiological conditions, increased levels of CD95L might prevent initial T-cell expansion. Respective scenarios have been reported for HIV,12 CMV35 and others. In fact, Raftery et al.35 observed that CMV-infected DCs not only down-modulate MHC molecules, but also upregulate CD95L, thereby inducing cell death of activated T cells and non-deletional suppression of the surviving T cells.
In conclusion, we suggest that, under physiological conditions, low-level CD95L on APC positively co-stimulates naïve T cells, allowing a first phase of expansion. During the immune response, CD95L is upregulated on APC and results in immune response termination by inducing apoptosis in pre-activated cells, and preventing the activation of additional effector cells. Under pathophysiological conditions, the threshold levels might be shifted to higher expression of CD95L, as a mechanism of immune evasion of certain pathogens.
Materials and Methods
Reagents and antibodies
TCR stimulation was performed with immobilized anti-CD3 mAb (OKT3, 2 μg/ml, IgG2a, Cilag, Sulzbach, Germany) and anti-CD28 mAb (CD28.2, 5 μg/ml, IgG1, BD Biosciences, San Diego, CA, USA). 96-well flat-bottom tissue culture (TC) plates were coated for 3 h at 37°C with 50 μl per well and 24-well TC plates with 250 μl of antibodies/fusion protein at the indicated concentrations in PBS. In some experiments, cells were stimulated with 10 ng/ml phorbolester PMA (Sigma, Munich, Germany) and 500 ng/ml ionomycin (Calbiochem, Darmstadt, Germany), or with PHA (PHA, 0.5 μg/ml, Remel, Dartford, UK). The anti-CD95 mAb 7C11 (IgM) was given to us by Michael J Robertson (Indianapolis, IN, USA). In most experiments, however, we used the agonistic anti-CD95 mAb anti-APO-1 (IgG3) generated in PH Krammer's laboratory.36 Human IgGFc was purchased from Bethyl Laboratories (Montgomery, TX, USA). Standard culture medium was X-VIVO (Lonza, Wuppertal, Germany). For some experiments, RPMI 1640 with glutamine, HEPES and antibiotics was used with 10% of pooled human AB serum (DRK, Baden–Baden, Germany) or 10% FBS (Biochrom, Berlin, Germany). Human recombinant interleukin-2 (rIL-2) (EuroCetus, Frankfurt, Germany), Protein A (Zymed Laboratories, South San Francisco, CA, USA), mistletoe lectin (Sigma), PD 98059 (Calbiochem), ZVAD(OMe)-fmk (Bachem, Weil am Rhein, Germany), LatA (Sigma) and cytochalasin D (Calbiochem) were added in some experiments as indicated.
Cells
PBMCs were isolated from buffy-coat preparations of healthy donors. CD4+ T cells were MACS-purified using negative selection kits from Miltenyi Biotec (Bergisch-Gladbach, Germany) or Dynal (Invitrogen, Karlsruhe, Germany). In some experiments the CD4+ T cells were further separated into CD45RA+ and CD45RO+ memory T cells using a depletion kit from Miltenyi Biotec. The purity of individual populations was examined by flow cytometry and reached routinely 95–99% of the desired phenotype. In some experiments, PHA-expanded T-cell blasts or Jurkat T cells (Je6.1) were used.
Flow cytometry
Purified T cells were incubated in untreated wells or in wells coated with anti-CD3 and anti-CD28 mAb in the presence or absence of co-stimulatory reagents as indicated. The cells were harvested and stained with fluorochrome-conjugated antibodies and analyzed using a FACS Calibur flow cytometer with Cellquest analysis software from Becton Dickinson. Changes in activation-dependent expression of surface molecules were tested with antibodies against CD95L (IgG1, Invitrogen), TCRα/β (MHAB01-4, IgG1, Caltag Laboratories, Hamburg, Germany), or CD25 (IL-2Rα, IgG1), CD69 (IgG1), CD122 (IL-2Rβ, IgG1), CD134 (OX-40, IgG1) and CD152 (CTLA-4, IgG2a) from BD Biosciences. For the identification of CD95 internalization, cells were stained with anti-APO-1 (1 μg per 106 cells), followed by incubation with PE-labeled goat-anti-mouse secondary antibodies (1 : 60, Invitrogen). Cell viability was assessed by dye exclusion using propidium iodide (PI) (PI, Serva, Heidelberg, Germany) at 2.5 μg/ml in PBS. For intracellular FACS analyses, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences). Cells were resuspended in Perm/Wash and incubated for 30 min at 4°C with fluorochrome-conjugated antibodies against IFNγ, TNFα or IL-4 from BD Biosciences.
Generation of fusion proteins
The cDNA sequence encoding the human Fc-fragment of IgG1 was cloned into a pCR3-derived human Flag-CD95L (CD95L, amino acids 95–282) protein expression vector provided by Harald Wajant (Würzburg, Germany). The Fc-Flag-CD95L pCR3 construct was modified by inserting a Strep-tag coding sequence, resulting in a Strep-Fc-Flag-CD95L pCR3 construct. Fusion proteins were generated as described.37 The leucine zipper LZ-CD95L was prepared as previously described.38
Proliferation assays: [3H]-thymidine (tdR) and BrdU incorporation, MTS assay and CFSE distribution assay
96-well flat-bottom TC plates were coated for 3 h at 37°C with 50 μl of OKT3, with or without anti-CD28. Anti-CD95 mAb (7C11 or anti-APO-1), CD95L fusion proteins or human IgGFc control protein were included in the coating solution as indicated. For the bromodeoxyuridine (BrdU) assay, we used the BrdU Labeling and Detection Kit III (Roche, Grenzach-Wyhlen, Germany). Stimulated cells (d2) were incubated with BrdU labeling reagent for 16 h. The incorporation of BrdU was determined by an ELISA using a POD-labeled anti-BrdU antibody. [3H]-TdR incorporation was measured as described before37. Alternatively, proliferation was analyzed using a colorimetric MTS/PMS assay (CellTiter96 AQueous Non-Radioactive Cell Proliferation Assay, Promega, Mannheim, Germany) according to the manufacturer's instructions. The optical densities of individual culture wells were read in a microplate reader at 490 nm. For the CFSE dilution assay, purified CD4+ T cells were labeled with 5-(6-)carboxyfluorescein diacetate succinimidyl ester (CFSE), and analyzed by flow cytometry.37
Western blot
For western blotting, we used antibodies against phospho-ERK, ERK, IκB, phospho-IκB, phospho-Rb (Ser795, Ser780 and Ser807/811), caspase-3 and phospho-STAT1 from New England Biolabs (Frankfurt, Germany), against CDK1, CDK2, CDK6, cyclin E, cyclin D2, cyclin B1, phospho-STAT4, PARP, Bcl-XL and PCNA from BD Biosciences, and against PLCγ, T-bet and cyclin D1 from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies against caspase-8 (C-15)39 and cFLIP (NF-6)40 were generated in the laboratory of PH Krammer.
Determination of caspase activity
A total of 106 CD4+ T cells were incubated for 3 days in 1 ml of culture medium in 24-well TC plates pretreated as indicated. For apoptosis induction, 106 Jurkat T cells (Je6.1) were incubated with 5 μg/ml of soluble CD95LFc for 2 h. Cells were washed with PBS and lysed in 35 μl lysis buffer (10 mM HEPES, 142 mM KCl, 5 mM MgCl2, 1 mM EDTA, 0.2% NP-40 and pH 7.2) with 10 mM DTT. Following incubation for 20 min on ice, samples were centrifuged and the protein content in supernatants was determined. Aliquots of 10 μg/100 μl assay volume were incubated with 140 μM site-specific tetrapeptide substrates (Ac-DEVD-AMC (7-amino-4-methylcoumarin) for caspase-3 and Ac-IETD-AMC for caspase-8 (Biomol, Hamburg, Germany)) in a caspase assay buffer (20 mM HEPES, 100 mM NaCl, 1 mM EDTA, 0.01% (w/v) CHAPS, 10% (w/v) sucrose and pH 7.2) with 10 mM DTT. The release of the fluorogenic group AMC was determined at 37°C in intervals of 3 min for at least 3 h on a microplate fluorescence reader (Fluoroscan II, Labsystems, Frankfurt, Germany) at 440–460 nm following excitation at 340–380 nm. Using an AMC calibration standard (AAT Bioquest, Sunnyvale, CA, USA), the caspase-3/-8 activity was quantified and calculated as pmol AMC substrate/min and pmol AMC/min/μg protein, respectively, according to the formula activity(pmol/min)=slope(abs/min) × conversion factor(μM/abs) × assay volume(μl).
Cell-cycle analysis, ATP assay and IL-2 ELISA
Primary CD4+ T cells (106/ml) were stimulated or not in 24-well TC plates coated with anti-CD3 mAb plus/minus anti-CD28 mAb with or without anti-CD95 mAb anti-APO-1 (5 μg/ml), CD95LFc or CD95L–ST–Fc fusion protein (2.5 or 20 μg/ml, respectively). Cell-cycle analysis was performed as described.15 The production of ATP was determined using the ViaLight Plus Kit (Lonza, Braine-l’Alleud, Belgium) according to the manufacturer's instructions. Luminescence was measured in a microplate reader. Supernatants from corresponding cultures were collected after 24, 48 and 72 h for assessment of IL-2 production by ELISA (R&D Systems, Wiesbaden, Germany).
Abbreviations
- abs:
-
absorbance
- AMC:
-
7-amino-4-methylcoumarin
- APC:
-
antigen-presenting cell
- Bcl-XL:
-
B-cell lymphoma-extra large
- BrdU:
-
bromodeoxyuridine
- CD95L:
-
CD95 ligand
- CDK:
-
cyclin-dependent kinase
- cFLIP:
-
cellular FLICE-inhibitory protein
- cFLIPS/R:
-
cFLIPshort/Raji
- cFLIPL:
-
cFLIPlong
- CFSE:
-
carboxy-fluorescein diacetate succinimidyl ester
- CTLA-4:
-
cytotoxic T-lymphocyte antigen-4
- CytD:
-
cytochalasin D
- DC:
-
dendritic cell
- DISC:
-
death-inducing signaling complex
- ERK:
-
extracellular signal-regulated protein kinase
- IFNγ:
-
interferon γ
- IκB:
-
inhibitor of NF-κB
- IL-2Rα/β:
-
interleukin-2 receptor α/β chain
- LatA:
-
latrunculin A
- LZ-CD95L:
-
leucin zipper CD95L
- mAb:
-
monoclonal antibody
- MAPK:
-
mitogen-activated protein kinase
- MFI:
-
mean fluorescence intensity
- NF-κB:
-
nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells
- PARP:
-
poly (ADP-ribose) polymerase
- PBMCs:
-
peripheral blood mononuclear cells
- PCNA:
-
proliferating cell nuclear antigen
- PD:
-
ERK1/2 inhibitor PD 98059
- PHA:
-
phytohemagglutinin
- PI:
-
propidium iodide
- PLCγ:
-
phospholipase Cγ
- PMA:
-
phorbol myristate acetate
- Rb:
-
retinoblastoma
- rIL-2:
-
recombinant IL-2
- TC:
-
tissue culture
- TCR:
-
T cell receptor
- TdR:
-
thymidine
- Th1:
-
T helper 1
- TNF:
-
tumor necrosis factor
- TNFR:
-
TNF receptor
- TRAF:
-
TNF receptor-associated factor
References
Frauwirth KA, Thompson CB . Activation and inhibition of lymphocytes by costimulation. J Clin Invest 2002; 109: 295–299.
Strasser A, Jost PJ, Nagata S . The many roles of FAS receptor signaling in the immune system. Immunity 2009; 30: 180–192.
Alderson MR, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K et al. Fas transduces activation signals in normal human T lymphocytes. J Exp Med 1993; 178: 2231–2235.
Kennedy NJ, Kataoka T, Tschopp J, Budd RC . Caspase activation is required for T cell proliferation. J Exp Med 1999; 190: 1891–1896.
Alam A, Cohen LY, Aouad S, Sekaly RP . Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med 1999; 190: 1879–1890.
Maksimow M, Soderstrom TS, Jalkanen S, Eriksson JE, Hanninen A . Fas costimulation of naive CD4T cells is controlled by NF-kappaB signaling and caspase activity. J Leukoc Biol 2006; 79: 369–377.
Kataoka T, Tschopp J . N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol 2004; 24: 2627–2636.
Golks A, Brenner D, Krammer PH, Lavrik IN . The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med 2006; 203: 1295–1305.
Krammer PH, Arnold R, Lavrik IN . Life and death in peripheral T cells. Nat Rev Immunol 2007; 7: 532–542.
Paulsen M, Ussat S, Jakob M, Scherer G, Lepenies I, Schütze S et al. Interaction with XIAP prevents full caspase-3/-7 activation in proliferating human T lymphocytes. Eur J Immunol 2008; 38: 1979–1987.
Wajant H, Pfizenmaier K, Scheurich P . Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 2003; 14: 53–66.
Strauss G, Lindquist JA, Arhel N, Felder E, Karl S, Haas TL et al. CD95 co-stimulation blocks activation of naive T cells by inhibiting T cell receptor signaling. J Exp Med 2009; 206: 1379–1393.
Crotzer VL, Mabardy AS, Weiss A, Brodsky FM . T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J Exp Med 2004; 199: 981–991.
Schütze S, Tchikov V, Schneider-Brachert W . Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol 2008; 9: 655–662.
Falk M, Ussat S, Reiling N, Wesch D, Kabelitz D, Adam-Klages S . Caspase inhibition blocks human T cell proliferation by suppressing appropriate regulation of IL-2, CD25, and cell cycle-associated proteins. J Immunol 2004; 173: 5077–5085.
Janssen O, Scheffler A, Kabelitz D . In vitro effects of mistletoe extracts and mistletoe lectins. Cytotoxicity towards tumor cells due to the induction of programmed cell death (apoptosis). Drug Research 1993; 43: 1221–1227.
Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK et al. The CD95 receptor: apoptosis revisited. Cell 2007; 129: 447–450.
Strauss G, Osen W, Knape I, Jacobsen EM, Muller SM, Debatin KM . Membrane-bound CD95 ligand expressed on human antigen-presenting cells prevents alloantigen-specific T cell response without impairment of viral and third-party T cell immunity. Cell Death Differ 2007; 14: 480–488.
Lavrik IN, Golks A, Riess D, Bentele M, Eils R, Krammer PH . Analysis of CD95 threshold signaling: triggering of CD95 (FAS/APO-1) at low concentrations primarily results in survival signaling. J Biol Chem 2007; 282: 13664–13671.
Alcover A, Alarcon B . Internalization and intracellular fate of TCR-CD3 complexes. Crit Rev Immunol 2000; 20: 325–346.
Chakrabandhu K, Huault S, Garmy N, Fantini J, Stebe E, Mailfert S et al. The extracellular glycosphingolipid-binding motif of Fas defines its internalization route, mode and outcome of signals upon activation by ligand. Cell Death Differ 2008; 15: 1824–1837.
Rossin A, Kral R, Lounnas N, Chakrabandhu K, Mailfert S, Marguet D et al. Identification of a lysine-rich region of Fas as a raft nanodomains targeting signal necessary for Fas-mediated cell death. Exp Cell Res 2010; 316: 1513–1522.
Shinohara H, Yagita H, Ikawa Y, Oyaizu N . Fas drives cell cycle progression in glioma cells via extracellular signal-regulated kinase activation. Cancer Res 2000; 60: 1766–1772.
Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol 2000; 10: 640–648.
Newton K, Strasser A . Caspases signal not only apoptosis but also antigen-induced activation in cells of the immune system. Genes Dev 2003; 17: 819–825.
Thome M, Tschopp J . TCR-induced NF-kappaB activation: a crucial role for Carma1, Bcl10 and MALT1. Trends Immunol 2003; 24: 419–424.
Zhang N, Hopkins K, He YW . c-FLIP protects mature T lymphocytes from TCR-mediated killing. J Immunol 2008; 181: 5368–5373.
Maksimow M, Santanen M, Jalkanen S, Hanninen A . Responding naive T cells differ in their sensitivity to Fas engagement: early death of many T cells is compensated by costimulation of surviving T cells. Blood 2003; 101: 4022–4028.
Cancedda C, LeMaoult J, Harris PE, Suciu-Foca N, Cortesini R . Specific T-cell deletion by transfected human monocytes expressing Fas ligand and antigen. Transplant Proc 2001; 33: 165–166.
Chen A, Zheng G, Tykocinski ML . Quantitative interplay between activating and pro-apoptotic signals dictates T cell responses. Cell Immunol 2003; 221: 128–137.
Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornard T et al. Activated B cells express functional Fas ligand. Eur J Immunol 1996; 26: 721–724.
Suss G, Shortman K . A subclass of dendritic cells kills CD4T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med 1996; 183: 1789–1796.
Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV . Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol 1996; 70: 199–206.
Hoves S, Krause SW, Herfarth H, Halbritter D, Zhang HG, Mountz JD et al. Elimination of activated but not resting primary human CD4+ and CD8+ T cells by Fas ligand (FasL/CD95L)-expressing Killer-dendritic cells. Immunobiology 2004; 208: 463–475.
Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G . Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 2001; 15: 997–1009.
Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989; 245: 301–305.
Paulsen M, Mathew B, Qian J, Lettau M, Kabelitz D, Janssen O . FasL cross-linking inhibits activation of human peripheral T cells. Int Immunol 2009; 21: 587–598.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–163.
Scaffidi C, Schmitz I, Krammer PH, Peter ME . The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 1999; 274: 1541–1548.
Scaffidi C, Medema JP, Krammer PH, Peter ME . FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J Biol Chem 1997; 272: 26953–26958.
Acknowledgements
This work forms part of the PhD thesis of M Paulsen. The project was sponsored by grants from the German Research Council (SFB 415, SFB 877) and the Medical Faculty of the Christian-Albrechts-University of Kiel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by H-U Simon
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Paulsen, M., Valentin, S., Mathew, B. et al. Modulation of CD4+ T-cell activation by CD95 co-stimulation. Cell Death Differ 18, 619–631 (2011). https://doi.org/10.1038/cdd.2010.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2010.134
Keywords
This article is cited by
-
The abscopal effect of anti-CD95 and radiotherapy in melanoma
Discover Oncology (2023)
-
Increased Expression of CD95 in CD4+ Effector Memory T Cells Promotes Th17 Response in Patients with Myasthenia Gravis
Journal of Neuroimmune Pharmacology (2022)
-
c-FLIP and CD95 signaling are essential for survival of renal cell carcinoma
Cell Death & Disease (2019)
-
Fas signaling-mediated TH9 cell differentiation favors bowel inflammation and antitumor functions
Nature Communications (2019)
-
A20 Curtails Primary but Augments Secondary CD8+ T Cell Responses in Intracellular Bacterial Infection
Scientific Reports (2016)