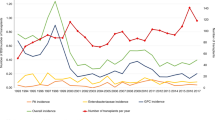
Abstract
Recent data link the incidence of intestinal GvHD (iGvHD) after allogeneic haematopoietic stem cell transplantation (aSCT) to exposure with piperacillin–tazobactam or imipenem–cilastatin. To assess relevance of timing, duration, sequence and combination of antibiotic treatment in this setting, we applied a time-dependent model to our aSCT cohort. Patients from the prospective Cologne Cohort of Neutropenic Patients (CoCoNut) undergoing aSCT from January 2007 to April 2013 were included into a time-dependent multivariate Cox proportional hazards regression model with backward-stepwise selection. In 399 eligible patients, cumulative antibiotic exposure (hazard ratio (HR) 2.46; 95% confidence interval (95% CI) 1.59–3.81; P<0.001) and exposure to sequential treatment with penicillin derivatives and carbapenems (HR 6.22, 95% CI 1.27–30.31), but not to the individual classes, were associated with iGvHD at day 100. Glycopeptides were assessed as a risk factor (HR 3.73, 95% CI 1.51–9.19), but not considered independent, since their use was dependent on previous exposure to penicillin derivatives and carbapenems. Patients with iGvHD presented with increased non-relapse mortality at day 365 (HR 3.51; 95% CI 2.10–5.89; P<0.001). We identified sequential exposure to penicillin derivatives and carbapenems as well as overall exposure to antibiotics as independent risk factors for iGVHD. Confirmation of these findings in larger, prospective cohorts is necessary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Taur Y, Pamer EG . Microbiome mediation of infections in the cancer setting. Genome Med 2016; 8: 40.
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8: 339ra371.
Weber D, Oefner PJ, Dettmer K, Hiergeist A, Koestler J, Gessner A et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant 2016; 51: 1087–1092.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C . Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44: 664–670.
Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012; 7: e51862.
R Core Team. R: A Language and Environment for Statistical Computing. 3.2.5 edn. R Foundation for Statistical Computing: Vienna, Austria, 2016.
Grambsch PM, Therneau TM . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526.
Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20: 640–645.
Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124: 1174–1182.
Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015; 21: 1373–1383.
Weber D, Oefner PJ, Hiergeist A, Koestler J, Gessner A, Weber M et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 2015; 126: 1723–1728.
Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 2016; 17: 505–513.
Weisser M, Theilacker C, Sutter ST, Babikir R, Bertz H, Gotting T et al. Secular trends of bloodstream infections during neutropenia in 15,181 haematopoietic stem cell transplants: 13-year results from a European Multicentre Surveillance Study (ONKO-KISS). Clin Microbiol Infect 2017.
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 2016; 27 (suppl 5): v111–v118.
Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013; 98: 1826–1835.
Paul M, Dickstein Y, Borok S, Vidal L, Leibovici L . Empirical antibiotics targeting Gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev 2014; 1: CD003914.
Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ . Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients—review of the literature from a clinical perspective. Crit Rev Microbiol 2016; 42: 1–16.
Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L . Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev 2013; 6: CD003038.
Paul M, Yahav D, Bivas A, Fraser A, Leibovici L . Anti-pseudomonal beta-lactams for the initial, empirical, treatment of febrile neutropenia: comparison of beta-lactams. Cochrane Database Syst Rev 2010; 11: CD005197.
Gyssens IC, Kern WV, Livermore DM,, Ecil ajvoEEI, ESCMID Eo. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematologica 2013; 98: 1821–1825.
Acknowledgements
We thank our medical documentation assistants Sandra Fuhrmann and Frank Müller for their help in electronic data capture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FF received travel grants from Astellas and MSD. MJGTV is a consultant to Berlin Chemie, MSD/Merck and Astellas Pharma; has served at the speakers’ bureau of Astellas Pharma, Basilea, Gilead Sciences, Merck/MSD, Organobalance and Pfizer; received research funding from 3M, Astellas Pharma, DaVolterra, Gilead Sciences, Merck/MSD, Organobalance and Seres Therapeutics. JJV is a consultant to Basilea, MSD/Merck and Astellas Pharma; has served at the speakers’ bureau of: Basilea, Pfizer, Merck/MSD, Gilead Sciences and Astellas Pharma; received research funding from Astellas Pharma, Basilea, Merck/MSD and Gilead Sciences. LMB has received lecture honoraria from Astellas and MSD and travel grants from 3M and Gilead. NJ received payments for lectures from MSD, travel grant from IMDx (Qiagen) and research funding from DaVolterra. OAC is supported by the German Federal Ministry of Research and Education and the European Commission, and has received research grants from, is an advisor to, or received lecture honoraria from, Actelion, Amplyx, Anacor, Aranis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, Da Volterra, Duke University (NIH UM1AI104681), F2G, Gilead, GSK, Janssen Pharmaceuticals, Leeds University, Matinas, MedPace, Melinta Therapeutics, Menarini Ricerche, Merck/MSD, Miltenyi, Paratek Pharmaceuticals, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres, Summit, The Medicine Company, Vical, Vifor. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Farowski, F., Bücker, V., Vehreschild, J. et al. Impact of choice, timing, sequence and combination of broad-spectrum antibiotics on the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 53, 52–57 (2018). https://doi.org/10.1038/bmt.2017.203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.203
This article is cited by
-
Roles of the intestinal microbiota and microbial metabolites in acute GVHD
Experimental Hematology & Oncology (2021)
-
Applications of gut microbiota in patients with hematopoietic stem-cell transplantation
Experimental Hematology & Oncology (2020)
-
Gut decontamination during allogeneic hematopoietic stem cell transplantation and the risk of acute graft-versus-host disease
Bone Marrow Transplantation (2018)