Abstract
This phase 1 study (clinical trial NCT00477815) was conducted to determine the maximum tolerated dose (MTD) of yttrium-90 ibritumomab tiuxetan (90Y-Zevalin) with high dose melphalan (HDM) therapy in multiple myeloma (MM) patients undergoing autologous stem cell transplantation (ASCT). In a 3+3 trial design, 30 patients received rituximab 250 mg/m2 with indium-111 ibritumomab tiuxetan (111In-Zevalin) for dosimetry (day −22); rituximab 250 mg/m2 with escalating doses of 90Y-Zevalin (day −14); melphalan 100 mg/m2 (days −2,−1) followed by ASCT (day 0) and sargramostim (GM-CSF, day 0) until neutrophil engraftment. Each patient’s 111In-Zevalin dosimetry data were used to calculate the dose of 90Y-Zevalin (in mCi) to deliver 10, 12, 14, 16, 18 or 20 Gy to the liver. Dose limiting toxicities were seen in 3 patients. The overall response rate was 73% (22/30) with stringent complete response in 2 patients; complete response, 5; very good partial response, 12; and partial response, 3. The median PFS was 16.5 months and the median overall survival was 63.4 months. In MM, the MTD of 90Y-Zevalin with HDM is 18 Gy to the liver. The addition of radiation with novel delivery methods such as radioimmunotherapy combined with standard transplant regimens warrants further study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Voorhees PM, Usmani SZ . The role of high-dose melphalan and autologous stem cell transplant in the rapidly evolving era of modern multiple myeloma therapy. Clin Adv Hematol Oncol 2016; 14: 719–728.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311–1320.
Gay F, Oliva S, Petrucci MT, Montefusco V, Conticello C, Musto P et al. Autologous transplant vs oral chemotherapy and lenalidomide in newly diagnosed young myeloma patients: a pooled analysis. Leukemia 2017; 31: 1727–1734.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371: 895–905.
Wildes TM, Finney JD, Fiala M, Gao F, Vij R, Stockerl-Goldstein K et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant 2015; 50: 1075–1082.
Muchtar E, Dingli D, Kumar S, Buadi FK, Dispenzieri A, Hayman SR et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone Marrow Transplant 2016; 51: 1449–1455.
Moreau P, Attal M, Harousseau JL . New developments in conditioning regimens before auto-SCT in multiple myeloma. Bone Marrow Transplant 2011; 46: 911–915.
Talamo G, Dimaio C, Abbi KK, Pandey MK, Malysz J, Creer MH et al. Current role of radiation therapy for multiple myeloma. Front oncol 2015; 5: 1–6.
Witzig TE . Radioimmunotherapy for patients with relapsed B-cell non-Hodgkin lymphoma. Cancer Chemother Pharmacol 2001; 48 (Suppl 1): S91–S95.
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN et al. Treatment With ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol 2002; 20: 3262–3269.
Witzig TE . Moving radioimmunotherapy forward for follicular lymphoma. J Clin Oncol 2013; 31: 294–296.
Kapoor P, Greipp PT, Morice WG, Rajkumar SV, Witzig TE, Greipp PR . Anti-CD20 monoclonal antibody therapy in multiple myeloma. Br J Haematol 2008; 141: 135–148.
Yavasoglu I, Sargin G, Kadikoylu G, Doger FK, Bolaman Z . Immunohistochemical evaluation of CD20 expression in patients with multiple myeloma. Rev Bras Hematol Hemoter 2015; 37: 34–37.
Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res 2008; 68: 190–197.
Hajek R, Okubote SA, Svachova H . Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol 2013; 163: 551–564.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Kaplan E, Meier P . Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood 2006; 107: 397–403.
Lonial S, Kaufman J, Tighiouart M, Nooka A, Langston AA, Heffner LT et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res 2010; 16: 5079–5086.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood 2010; 115: 32–37.
Reece D, Song K, LeBlanc R, Mezzi K, Olujohungbe A, White D et al. Efficacy and safety of busulfan-based conditioning regimens for multiple myeloma. Oncologist 2013; 18: 611–618.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood 2002; 99: 731–735.
Dispenzieri A, Wiseman GA, Lacy MQ, Litzow MR, Anderson PM, Gastineau DA et al. A phase I study of 153Sm-EDTMP with fixed high-dose melphalan as a peripheral blood stem cell conditioning regimen in patients with multiple myeloma. Leukemia 2005; 19: 118–125.
Christoforidou AV, Saliba RM, Williams P, Qazilbash M, Roden L, Aleman A et al. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant 2007; 13: 543–549.
Stevens PL, Oluwole O, Reddy N . Advances and application of radioimmunotherapy in non-Hodgkin lymphoma. Am J Blood Res 2012; 2: 86–97.
Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood 2003; 102: 1070–1071.
Kumar S, Kimlinger T, Morice W . Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol 2010; 23: 433–451.
Cremer FW, Goldschmidt H, Moos M . Clonotypic B cells in the peripheral blood of patients with multiple myeloma. Blood 2001; 97: 2913–2914.
Dolai TK, Nataraj KS, Bhattacharya M, Ghosh MK . Veno-occlusive disease following high dose melphalan. Indian j hematol blood transfusion 2012; 28: 62–63.
Labidi SI, Sebban C, Ghesquieres H, Nicolas EV, Biron P . Hepatic veno-occlusive disease after tandem autologous stem cell transplantation conditioned by melphalan. Int J Hematol 2008; 88: 291–293.
Kumar S, DeLeve LD, Kamath PS, Tefferi A . Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc 2003; 78: 589–598.
Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant 2013; 19: 760–766.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207–1219.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551–1560.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 2014; 89: 926–933.
Acknowledgements
We thank the RN transplant coordinators—Teresa Miceli, Joan Theuer, Marsha Knudsvig, Michelle Gronseth and Lisa Kaiser for their assistance in patient accrual. We are extremely grateful for the philanthropic support provided by a gift from Hank Asher, which was paramount in our work to advance the treatment of multiple myeloma—an ongoing focus of investigation at Mayo Clinic for over 40 years.
Author contributions
AD, ADS and TEW contributed to the study design, data analysis and collection, care of patients, writing and edited the manuscript. KL contributed to data analysis and collection and the writing and editing of the manuscript. GW assisted with study design, data collection, care of patients and manuscript editing. BL contributed to the study design, data analysis and collection and manuscript editing. MQL, FB, SRH, SKK, DD, WJH, SMA, DAG, DJI, INM, LFP, PBJ, MRL all contributed to data collection, care of patients and editing of the manuscript. MAG provided care of patients and manuscript editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented at the 2011 ASH Annual Meeting, San Diego, CA, USA. Blood (ASH Annual Meeting Abstracts) 2011; 118: Abstract 3095. This trial is registered at https://clinicaltrials.gov as NCT00477815.
Rights and permissions
About this article
Cite this article
Dispenzieri, A., D'Souza, A., Gertz, M. et al. A phase 1 trial of 90Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma. Bone Marrow Transplant 52, 1372–1377 (2017). https://doi.org/10.1038/bmt.2017.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.164
This article is cited by
-
Use of imaging-based dosimetry for personalising radiopharmaceutical therapy of cancer
Cancer Imaging (2022)
-
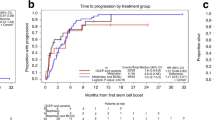
Success of the autologous stem cell boost after autologous graft failure in multiple myeloma and AL amyloidosis
Bone Marrow Transplantation (2022)