Abstract
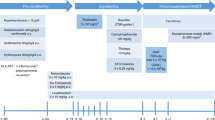
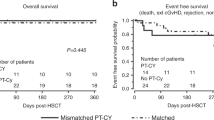
Thalassemia-free survival after allogeneic stem cell transplantation (SCT) is about 80–90% with either matched-related or -unrelated donors. We explored the use of a mismatched-related (‘haplo- ’) donor. All patients received two courses of pretransplant immunosuppressive therapy (PTIS) with fludarabine (Flu) and dexamethasone (Dxm). After two courses of PTIS, a conditioning regimen of rabbit antithymocyte globulin, Flu and IV busulfan (Bu) was given followed by T-cell-replete peripheral blood progenitor cells. GvHD prophylaxis consisted of cyclophosphamide (Cy) on days SCT +3 and +4 (post-Cy), and on day SCT +5 tacrolimus or sirolimus was started together with a short course of mycophenolate mofetil. Thirty-one patients underwent haplo-SCT. Their median age was 10 years (range, 2–20 years). Twenty-nine patients engrafted with 100% donor chimerism. Two patients suffered primary graft failure. Median time to neutrophil engraftment was 14 days (range, 11–18 days). Five patients developed mild to moderate, reversible veno-occlusive disease, while nine patients developed acute GvHD grade II. Only five patients developed limited-chronic GvHD. Projected overall and event-free survival rates at 2 years are 95% and 94%, respectively. The median follow up time is 12 months (range, 7–33 months).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR et al. Survival and complications in thalassemia. Ann N Y Acad Sci 2005; 1054: 40–47.
Lucarelli G, Clift RA, Galimberti M, Polchi P, Angelucci E, Baronciani D et al. Marrow transplantation for patients with thalassemia: results in class 3 patients. Blood 1996; 87: 2082–2088.
Angelucci E, Matthes-Martin S, Baroninciani D, Bernaudin F, Bonanoni S, Cappelleni MD et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica 2014; 99: 811–820.
Leelahavarong P, Chaikledkaew U, Hongeng S, Kasemsup V, Lubell Y, Teerawattananon Y . A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res 2010; 10: 209–220.
Sruamsiri R, Chaiyakunapruk N, Pakakasama S, Sirireung S, Sripaiboonkij N, Bunworasate U et al. Cost utility analysis of reduced intensity hematopoietic stem cell transplantation in adolescence and young adult with severe thalassemia compared to hypertransfusion and iron chelation program. BMC Health Serv Res 2013; 13: 45–57.
Sodani P, Gaziev D, Polchi P, Erer B, Giardini C, Angelucci E et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood 2004; 104: 1201–1203.
Gaziev J, Nguyen L, Puozzo C, Mozzi AF, Casella M, Perrone Donnorso M et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamics profile with therapeutic drug monitoring. Blood 2010; 115: 4597–4604.
Chiesa R, Capelli B, Crocchiolo R, Frugnoli L, Biral E, Noe A et al. Unpredictability of intravenous busulfan pharmacokinetics in children undergoing hematopoietic stem cell transplantation for advanced beta thalassemia: limited toxicity with a dose-adjustment policy. Biol Blood Marrow Transplant 2010; 16: 622–628.
Mathews V, George B, Deotare U, Lakshmi KM, Viswabandya A, Daniel D et al. A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with beta thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 889–894.
Anurathapan U, Pakakasama S, Rujkijyanont P, Sirachainan N, Songdej D, Chuansumrit A et al. Pharmacologic Immunoablation followed by reduced-toxicity conditioning and stem cell transplantation in high-risk thalassemi: a safe approach to disease control. Biol Blood Marrow Transplant 2013; 19: 1259–1262.
Anurathapan U, Pakakasama S, Mekjaruskul P, Sirachainan N, Songdej D, Chuansumrit A et al. Outcomes of thalassemia patients undergoing hematopoietic stem cell transplant by using a standard myeloablative versus a novel reduced toxicity conditioning regimen according to new risk stratification. Biol Blood Marrow Transplant 2014; 20: 2066–2071.
Luznik L, O’Donnell PV, Fuchs EJ . Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 2012; 39: 683–693.
Kanakry JA, Kasamon YL, Gocke CD, Tsai HL, Davis-Sproul J, Ghosh N et al. Outcomes of related donor HLA-identical or HLA-haploidentical allogeneic blood or marrow transplantation for peripheral T cell lymphoma. Biol Blood Marrow Transplant 2013; 19: 602–606.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 2014; 32: 3497–3505.
Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-compatible BMT for AML, ALL, and MDS. Blood 2014; 124: 3817–3827.
Raiola AM, Dominietto A, di Grazia C, Gualandi F, Ibatici A, Bregante S et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 2014; 20: 1573–1579.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012; 120: 4285–4291.
Sodani P, Isgro A, Gaziev J, Polchi P, Paciaroni K, Marziali M et al. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood 2010; 115: 1296–1302.
Isgro A, Marziali M, Sodani P, Gaziev J, Erer B, Polchi P et al. Immunohematologic reconstitution in pediatric patients after T cell-depleted HLA-haploidentical stem cell transplantation for thalassemia. Biol Blood Marrow Transplant 2010; 16: 1557–1566.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
De Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Sabloff M, Chandy M, Wang Z, Logan BR, Ghavamzadeh A, Li CK et al. HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood 2011; 117: 1745–1750.
Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H . Fetal-maternal microchimerism: impact on hematopoietic stem cell transplantation. Curr Opin Immunol 2005; 17: 546–552.
Patriarca F, Luznik L, Medeot M, Zecca M, Bacigalupo A, Di Bartolomeo P et al. Experts’ considerations on HLA-haploidentical stem cell transplantation. Eur J Haematol 2014; 93: 187–197.
Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood 2011; 118: 6691–6697.
Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant 2012; 47: 508–515.
Socie G, Lawler M, Gluckman E, McCann SR, Brison O . Studies on hemopoietic chimerism following allogeneic bone marrow transplantation in the molecular biology era. Leuk Res 1995; 19: 497–504.
Kaplan EL, Meier P . Nonparametric estimator from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Venoocclusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Bearman SI . The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005–3020.
Chandy M, Balasubramanian SV, Ramachandran SV, Mathews V, George B, Dennison D et al. Randomized trial of two different conditioning regimens for bone marrow transplantation in thalassemia—the role of busulfan pharmacokinetics in determining outcome. Bone Marrow Transplant 2005; 36: 839–845.
Bernado ME, Piras E, Vacca A, Giorgiani G, Zecca M, Bertaina A et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 2012; 120: 473–476.
Mathews V, George B, Viswabandya A, Abraham A, Ahmed R, Ganapule A et al. Improved clinical outcomes of high risk beta thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS ONE 2013; 8: e61637.
Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia 2015; 29: 1891–1900.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X et al. Haploidentical- versus identical-sibling transplant for AML in remission: a multi-centre, prospective study. Blood 2015; 125: 3956–3962.
Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R . Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos 2001; 29: 264–267.
Malar R, Sjoo F, Rentsch K, Hassan M, Gungor T . Therapeutic drug monitoring is essential for intravenous busulfan therapy in pediatric hematopoietic stem cell recipients. Pediatr Transplant 2011; 15: 580–588.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Andersson BS, de Lima MJ, Saliba RM, Shpall EJ, Popat U, Jones R et al. Pharmacokinetic dose guidance of IV busulfan with fludarabine with allogeneic stem cell transplantation improves progression free survival in patients with AML and MDS; results of a randomized phase III study. Blood 2011; 118: 892.
Popat U, Bassett R, Chen J, Alousi AM, Anderlini P, Ciurea SO et al. Allogeneic transplantation for myelofibrosis: final analysis of a prospective study after a median follow up of 5 years. J Clin Oncol 2015; 33 (suppl; abstr 7008)..
Furst S, Bernit E, El Cheikh J, Granata A, Harbi S, Mohty B et al. Successful engraftment and clearance of donor specific antibodies after haploidentical related stem cell transplantation for an adult patient with sickle cell disease. Blood 2014; 124: 5792.
Acknowledgements
This work was supported in part by grants from the Ramathibodi Foundation, the National Research University Grant, the Mahidol University Research Grant, the Office of the Higher Education Commission and Mahidol University under the National Research University Initiative, Thailand Research Fund, Research Chair Grant from the National Science and Technology Development Agency (NSTDA) and the National Institutes of Health of United States (CCSG CA16672).
Author contributions
SH and BSA designed the research. SH, SP and UA performed the research. SH, UA, RS and BSA analyzed the data and performed the statistical analyses. SH, SP, UA, RS and BSA wrote the manuscript. NS, DS, AC, PC, AJ, KS, PR, AM, YL, PI, PS, WS, SS, PA, AU and SI enrolled the patients.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anurathapan, U., Hongeng, S., Pakakasama, S. et al. Hematopoietic stem cell transplantation for homozygous β-thalassemia and β-thalassemia/hemoglobin E patients from haploidentical donors. Bone Marrow Transplant 51, 813–818 (2016). https://doi.org/10.1038/bmt.2016.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.7
This article is cited by
-
Durable engraftment after pharmacological pre-transplant immune suppression followed by reduced-toxicity myeloablative haploidentical stem cell transplantation in highly HLA-immunized adults with sickle cell disease
Bone Marrow Transplantation (2024)
-
Health state utilities for beta-thalassemia: a time trade-off study
The European Journal of Health Economics (2023)
-
Busulfan–fludarabine- or treosulfan–fludarabine-based myeloablative conditioning for children with thalassemia major
Annals of Hematology (2022)
-
Pre-transplant myeloid and immune suppression, upfront plerixafor mobilization and post-transplant cyclophosphamide: novel strategy for haploidentical transplant in sickle cell disease
Bone Marrow Transplantation (2021)
-
No differences in hemostatic and endothelial activations between haploidentical and matched-donor hematopoietic stem cell transplantation in thalassemia disease
Thrombosis Journal (2020)