Abstract
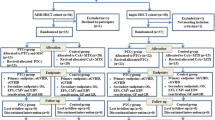
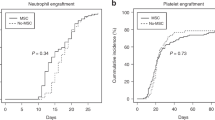
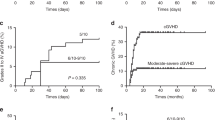
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) for severe aplastic anemia (SAA) is mainly limited by the high incidence of graft failure and GvHD. Mesenchymal stem cells (MSCs) have been shown to support hematopoiesis in vivo and to display potent immunosuppressive effects to prevent or treat GvHD after HSCT. In a multicenter phase II trial, we developed an approach with co-transplantation of MSCs in patients undergoing haplo-HSCT. Forty-four patients with SAA were included. The conditioning regimen included busulfan, cyclophosphamide and thymoglobulin (ATG). The recipients received cyclosporin A (CsA), mycophenolate mofetil and short-term methotrexate for GvHD prophylaxis. Three out of 44 patients, who died early before hematopoietic engraftment, were not assessed. Evaluable patients (97.6%; 40/41) achieved hematopoietic reconstitution and sustained full donor chimerism. The median time for myeloid engraftment was 12 days (range 8–21 days) and for platelet engraftment was 19 days (range 8–154 days). The incidence was 29.3% for grade II–IV acute GvHD and 14.6% for chronic GvHD. The overall survival was 77.3% with a median 12-month (range 0.9–30.8) follow-up for surviving patients. These data suggest that co-transplantation of MSCs could reduce the risk of graft failure and severe GvHD in haplo-HSCT for SAA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297.
González-Vicent M, Molina B, Andión M, Sevilla J, Ramirez M, Pérez A et al. Allogeneic hematopoietic transplantation using haploidentical donor vs unrelated cord blood donor in pediatric patients: a single-center retrospective study. Eur J Haematol 2011; 87: 46–53.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Lacerda JF, Martins C, Carmo JA, Lourenco F, Juncal C, Ismail S et al. Haploidentical stem cell transplantation with purified CD34+ cells after a chemotherapy-alone conditioning regimen in heavily transfused severe aplastic anemia. Biol Blood Marrow Transplant 2005; 11: 399–400.
Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E et al. Effective donor lymphohematopoietic reconstitution after haplo-identical CD34+/− selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Guo M, Sun Zh, Sun QY, Han Q, Yu CL, Wang DH et al. A modified haploidentical nonmyeloablative transplantation without T cell depletion for high-risk acute leukemia: successful engraftment and mild GVHD. Biol Blood Marrow Transplant 2009; 15: 930–937.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I . The role of mesenchymal stem cells in haemopoiesis. Blood Rev 2006; 20: 161–171.
Xiao Y, Jiang ZJ, Pang Y, Li L, Gao Y, Xiao HW et al. Efficacy and safety of mesenchymal stromal cell treatment from related donors for patients with refractory aplastic anemia. Cytotherapy 2013; 15: 760–766.
Wang H, Wang Z, Zheng X, Ding L, Yan H, Guo Z . Hematopoietic stem cell transplantation with umbilical cord multipotent stromal cell infusion for the treatment ofaplastic anemia—a single-center experience. Cytotherapy 2013; 15: 1118–1125.
Fu Y, Wang Q, Zhou J, Liu S, Fang B, Wei X et al. Reduced intensity conditioning and co-transplantation of unrelated peripheral stem cells combined with umbilicalcord mesenchymal stem/stroma cells for young patients with refractory severe aplastic anemia. Int J Hematol 2013; 98: 658–663.
Li XH, Gao CJ, Da WM, Cao YB, Wang ZH, Xu LX et al. Reduced intensity conditioning,combined transplantation of haploidentical hematopoietic stem cells andmesenchymal stem cells in patients with severe aplastic anemia. PLoS ONE 2014; 9: 1–7.
Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P et al. Contransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplasticanemia: successful engraftment and mild GVHD. Stem Cell Res 2014; 12: 132–138.
Li L, Pang Y, Zhang H, Kuang LP, Xiao ZF, Wang YC et al. Human umbilical cord mesenchymal stem cells cultured in serum-decremental medium in vitro. Chinese J Tissue Eng Res 2012; 16: 989–993.
Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J et al. Treatment of acquired severe aplastic anaemia: bone marrow transplantation compared with immunosuppressive therapy - The European Group for Blood and Marrow Transplantation experience. Semin Hematol 2000; 37: 69–80.
Fuhrer M . Risk-adapted procedures for HSCT from alternative donor in children with severe aplastic anemia. Bone Marrow Transplant 2008; 42 (S2): S97–S100.
Xiao Y, Song JY, Jiang ZJ, Li YH, Gao Y, Xu WN et al. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci 2014; 11: 652–657.
Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia 2009; 23: 78–84.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Luo XH, Chang YJ, Huo MR, Li D, Huang XJ . Cytomegalovirus-specific T cells immune reconstitution after human leukocyte antigen matched sibling donor allogeneic bone marrow plus peripheral blood hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2012; 33: 605–609.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
Han TT, Xu LP, Liu DH, Liu KY, Zhang XH, Chen H et al. Prevalence of EBV infection in patients with allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2013; 34: 651–654.
de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012; 367: 2305–2315.
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC . Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389–397.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838–3843.
Aggarwal S, Pittenger MF . Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822.
Dander E, Lucchini G, Vinci P, Introna M, Bonanomi S, Balduzzi A et al. Understanding the immunomodulatory effect of mesenchymal stem cell infused in transplanted patients with steroid-refractory GVHD. Blood (ASH Annu Meet Abstracts) 2010; 116: 2306.
Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007; 21: 1733–1738.
Fang B, Song Y, Li N, Li J, Zhao RC . Cotransplantation of haploidentical mesenchymal stem cells to reduce the risk of graft failure in a patient with refractory severe aplastic anemia. Acta Haematol 2008; 119: 162–165.
Fang B, Li N, Song Y, Li J, Zhao RC, Ma Y . Cotransplantation of haploidentical mesenchymal stem cells to enhance engraftment of hematopoietic stem cells and to reduce the risk of graft failure in two children with severe aplastic anemia. Pediatr Transplant 2009; 13: 499–502.
Jaganathan BG, Tisato V, Vulliamy T, Dokal I, Marsh J, Dazi F et al. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia 2010; 24: 1791–1795.
Wang H, Yan H, Wang Z, Zhu L, Liu J, Guo Z . Cotransplantation of allogeneic mesenchymal and hematopoietic stem cells in children with aplastic anemia. Pediatrics 2012; 129: e1612–e1615.
Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res 2014; 12: 132–138.
Fu Y, Wang Q, Zhou J, Liu S, Fang B, Wei X et al. Reduced intensity conditioning and co-transplantation of unrelated peripheral stem cells combined with umbilical cord mesenchymal stem/stroma cells for young patients with refractory severe aplastic anemia. Int J Hematol 2013; 98: 658–663.
Li XH, Gao CJ, Da WM, Cao YB, Wang ZH, Xu LX et al. Reduced intensity conditioning, combined transplantation of haploidentical hematopoietic stem cells and mesenchymal stem cells in patients with severe aplastic anemia. PLoS ONE 2014; 9: 1–7.
Wu YM, Cao YB, Li XH, Xu LX, Liu ZY, Liu B et al. Clinical effect of cotransplantation of haploidentical hematopoietic stem cells and umbilical cord mesenchymal stem cells for 27 patients with severe aplastic anemia. Jie Fang Jun Yi Xue Yuan Xue Bao 2014; 35: 1191–1195.
Zhang C, Huang X, Liu D, Mo X, Zhang X, Chen H et al. The risk factors of post-transplant lymphoproliferative disorders following haploidentical hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi 2014; 53: 527–531.
Xu LP, Huang XJ, Liu DH, Chen YH, Shi HX, Chen DB . A clinical study of lymphoproliferative disorders following allogeneic hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi 2007; 46: 996–999.
Lin Z, KongY, Wang Y, Zhang X, Liu D, Xu L et al. Analysis of risk factors for secondary cytopenia after allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2014; 35: 4–8.
Fu YW, de Wu P, Cen JN, Feng YF, Chang WR, Zhu ZL et al. Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients-a study in Chinese patients. Eur J Haematol 2007; 79: 138–145.
Sayer HG, Longton G, Bowden R, Pepe M, Storb R . Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood 1994; 84: 1328–1332.
Acknowledgements
This work was supported by funding from the Guangzhou Health and Medical Collaborative Innovation Major Program, Project number 201400000003-1 and 201400000003-4; the Army Medical, Science and Technology Research Program (12.5 Program), Project number BWS11J071; the State Natural Sciences Fund, Project number 81570107; and Natural Science Foundation of Guangdong Province, Project number 2014A030311006.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, Y., Xiao, H. et al. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transplant 52, 704–710 (2017). https://doi.org/10.1038/bmt.2016.347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.347
This article is cited by
-
A review of the application of mesenchymal stem cells in the field of hematopoietic stem cell transplantation
European Journal of Medical Research (2023)
-
Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?—a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Combination of haploidentical haematopoietic stem cell transplantation with an unrelated cord-blood unit in patients with severe aplastic anemia: a report of 146 cases
Bone Marrow Transplantation (2020)
-
Current insights into the treatments of severe aplastic anemia in China
International Journal of Hematology (2020)
-
Donor-derived marrow mesenchymal stromal cell co-transplantation following a haploidentical hematopoietic stem cell transplantation trail to treat severe aplastic anemia in children
Annals of Hematology (2019)