Abstract
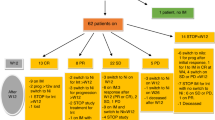
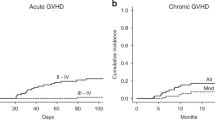
A nationwide retrospective study for the clinical outcomes of 99 patients who had received thymoglobulin at a median total dose of 2.5 mg/kg (range, 0.5–18.5 mg/kg) as a second-line treatment for steroid-resistant acute GvHD was conducted. Of the 92 evaluable patients, improvement (complete or partial response) was observed in 55 patients (60%). Multivariate analysis demonstrated that male sex and grade III and IV acute GvHD were associated with a lower improvement rate, whereas thymoglobulin dose (<2.0, 2.0–3.9 and ⩾4.0 mg/kg) was NS. Factors associated with significantly higher nonrelapse mortality included higher patient age (⩾50 years), grade IV acute GvHD, no improvement of GvHD and higher dose of thymoglobulin (hazard ratio, 2.55; 95% confidence interval, 1.34–4.85; P=0.004 for 2.0–3.9 mg/kg group and 1.79; 0.91–3.55; P=0.093 for ⩾4.0 mg/kg group). Higher dose of thymoglobulin was associated with a higher incidence of bacterial infections, CMV antigenemia and any additional infection. Taken together, low-dose thymoglobulin at a median total dose of 2.5 mg/kg provides a comparable response rate to standard-dose thymoglobulin reported previously, and <2.0 mg/kg thymoglobulin is recommended in terms of the balance between efficacy and adverse effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990; 75: 1024–1030.
Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood 1990; 76: 1464–1472.
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012; 18: 1150–1163.
MacMillan ML, DeFor TE, Weisdorf DJ . The best endpoint for acute GVHD treatment trials. Blood 2010; 115: 5412–5417.
Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2013; 19: 1183–1189.
McCaul KG, Nevill TJ, Barnett MJ, Toze CL, Currie CJ, Sutherland HJ et al. Treatment of steroid-resistant acute graft-versus-host disease with rabbit antithymocyte globulin. J Hematother Stem Cell Res 2000; 9: 367–374.
Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant 2001; 27: 1059–1064.
MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NK et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant 2002; 8: 40–46.
Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB . Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant 2002; 8: 155–160.
Graziani F, Van Lint MT, Dominietto A, Raiola AM, Di Grazia C, Lamparelli T et al. Treatment of acute graft versus host disease with low dose-alternate day anti-thymocyte globulin. Haematologica 2002; 87: 973–978.
Ohashi K, Tanaka Y, Mori S, Okuyama Y, Hiruma K, Akiyama H et al. Low-dose antithymocyte globulin for treatment of steroid-pulse-resistant acute graft-versus-host disease. Int J Hematol 2003; 77: 99–102.
Van Lint MT, Milone G, Leotta S, Uderzo C, Scimè R, Dallorso S et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood 2006; 107: 4177–4181.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol 2007; 86: 269–274.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J Royal Stat Soc Series B 1972; 34: 187–220.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15: 367–369.
Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant 2003; 9: 460–471.
Seidel MG, Fritsch G, Matthes-Martin S, Lawitschka A, Lion T, Pötschger U et al. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2005; 27: 532–536.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2015; 2: e194–e203.
Storek J, Mohty M, Boelens JJ . Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2015; 21: 959–970.
Mohty M . Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21: 1387–1394.
Xhaard A, Moins-Teisserenc H, Busson M, Robin M, Ribaud P, Dhedin N et al. Reconstitution of regulatory T-cell subsets after allogeneic hematopoietic SCT. Bone Marrow Transplant 2014; 49: 1089–1092.
Acknowledgements
We would like to thank the physicians at each transplantation center and the data manager at the Japanese Data Center for Hematopoietic Cell Transplantation. This study was supported in part by a grant from the Japan Society for the Promotion of Science (JSPS) (15K09498 to MM) and the Japan Agency for Medical Research and Development (AMED) (15ek0510010h0003 to MM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Appendix
Appendix
Institutes participating in this study: Hokkaido University Hospital; Sapporo Hokuyu Hospital; Sapporo City General Hospital; Aomori Prefectural Central Hospital; Iwate Medical University; University of Tsukuba Hospital; Jichi Medical University Hospital; Gunmaken Saiseikai Maebashi Hospital; Saitama Medical Center; Saitama Medical Center, Jichi Medical University; National Cancer Center Hospital; The Jikei University; Keio University Hospital; Toranomon Hospital; Medical Hospital, Tokyo Medical and Dental University; Kanagawa Cancer Center; Tokai University School of Medicine; Kanagawa Children’s Medical Center; Yokohama Municipal Citizen’s Hospital; Niigata University Medical & Dental Hospital; Toyama Prefectural Central Hospital; Shizuoka Cancer Center; Nagoya University Hospital; Nagoya Medical Center; Aichi Medical University Hospital; Osaka Medical Center for Cancer and Cardiovascular Diseases; Kinki University Hospital, Faculty of Medicine; Osaka University hospital; Osaka City University Hospital; Osaka City General Hospital; Osaka Medical Center and Research Institute for Maternal and Child Health; Matsushita Memorial Hospital; Sakai Hospital Kinki University, Faculty of Medicine; Hirakata Kohsai Hospital; Hyogo College of Medicine; Hyogo Cancer Center; Shimane Prefectural Central Hospital; Okayama University Hospital; Kawasaki Medical School Hospital; Hiroshima University Hospital; Tokushima Red Cross Hospital; Kochi Medical School Hospital; Kyushu University Hospital; Harasanshin Hospital; Hamanomachi Hospital; St Mary’s Hospital; Kurume University Hospital; National Kyushu Medical Center; Kitakyushu Municipal Medical Center; Nagasaki University Hospital; Oita University Hospital; Oita Prefectural Hospital.
Rights and permissions
About this article
Cite this article
Murata, M., Ikegame, K., Morishita, Y. et al. Low-dose thymoglobulin as second-line treatment for steroid-resistant acute GvHD: an analysis of the JSHCT. Bone Marrow Transplant 52, 252–257 (2017). https://doi.org/10.1038/bmt.2016.247
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.247
This article is cited by
-
Understanding and treatment of cutaneous graft-versus-host-disease
Bone Marrow Transplantation (2023)
-
Letter to the Editor: Very low-dose antithymocyte globulin (thymoglobulin) is effective for steroid-refractory acute graft-versus-host disease involving the skin or gut after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)
-
Off-the-shelf bone marrow-derived mesenchymal stem cell treatment for acute graft-versus-host disease: real-world evidence
Bone Marrow Transplantation (2021)
-
Antithymocyte globulin
Reactions Weekly (2017)