Abstract
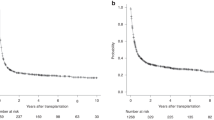
In the CORAL study, 255 chemosensitive relapses with diffuse large B-cell lymphoma (DLBCL) were consolidated with autologous stem cell transplantation (ASCT), and 75 of them relapsed thereafter. The median time between ASCT and progression was 7.1 months. The median age was 56.1 years; tertiary International Prognosis Index (tIPI) observed at relapse was 0–2 in 71.6% of the patients and >2 in 28.4%. The overall response rate to third-line chemotherapy was 44%. The median overall survival (OS) was 10.0 months (median follow-up: 32.8 months). Thirteen patients received an allogeneic SCT, and three a second ASCT. The median OS was shorter among patients who relapsed <6 months (5.7 months) compared with those relapsing ⩾12 months after ASCT (12.6 months, P=0.0221). The median OS in patients achieving CR, PR or no response after the third-line regimen was 37.7 (P<0.0001), 10.0 (P=0.03) and 6.3 months, respectively. The median OS varied according to tIPI: 0–2: 12.6 months and >2: 5.3 months (P=0.0007). In multivariate analysis, tIPI >2, achievement of response and remission lasting <6 months predicted the OS. This report identifies the prognostic factors for DLBCL relapsing after ASCT and thus helps to select patients for experimental therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–4190.
Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol 2012; 30: 4462–4469.
Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 2015; 51: 51–57.
Nagle SJ, Woo K, Schuster SJ, Nasta SD, Stadtmauer E, Mick R et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 2013; 88: 890–894.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244.
Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 2011; 29: 4079–4087.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–282.
Vose JM, Bierman PJ, Anderson JR, Kessinger A, Pierson J, Nelson J et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood 1992; 80: 2142–2148.
Robinson SP, Boumendil A, Finel H, Blaise D, Poire X, Nicolas-Virelizier E et al. Autologous stem cell transplantation for relapsed/refractory diffuse large B-cell lymphoma: efficacy in the rituximab era and comparison to first allogeneic transplants. A report from the EBMT Lymphoma Working Party. Bone Marrow Transplant 2015; 51: 365–371.
Redondo AM, Pomares H, Vidal MJ, Pascual MJ, Quereda B, Sancho JM et al. Impact of prior rituximab on outcomes of autologous stem-cell transplantation in patients with relapsed or refractory aggressive B-cell lymphoma: a multicentre retrospective Spanish group of lymphoma/autologous bone marrow transplant study. Br J Haematol 2014; 164: 668–674.
van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol 2011; 29: 1342–1348.
Rigacci L, Puccini B, Dodero A, Iacopino P, Castagna L, Bramanti S et al. Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Ann Hematol 2012; 91: 931–939.
Sirvent A, Dhedin N, Michallet M, Mounier N, Faucher C, Yakoub-Agha I et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant 2010; 16: 78–85.
Bacher U, Klyuchnikov E, Le-Rademacher J, Carreras J, Armand P, Bishop MR et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood 2012; 120: 4256–4262.
Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol 2008; 19: 1935–1940.
Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol 2014; 15: 757–766.
Rezvani AR, Kanate AS, Efron B, Chhabra S, Kohrt HE, Shizuru JA et al. Allogeneic hematopoietic cell transplantation after failed autologous transplant for lymphoma using TLI and anti-thymocyte globulin conditioning. Bone Marrow Transplant 2015; 50: 1286–1292.
Kim JW, Kim SW, Tada K, Fukuda T, Lee JH, Lee JJ et al. Allogeneic stem cell transplantation in patients with de novo diffuse large B-cell lymphoma who experienced relapse or progression after autologous stem cell transplantation: a Korea-Japan collaborative study. Ann Hematol 2014; 93: 1345–1351.
Avivi I, Canals C, Vernant JP, Wulf G, Nagler A, Hermine O et al. Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplant 2014; 49: 671–678.
Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol 2016; 174: 235–248.
Mondello P, Younes A . Emerging drugs for diffuse large B-cell lymphoma. Expert Rev Anticancer Ther 2015; 15: 439–451.
Nowakowski GS, Czuczman MS . ABC, GCB, and double-hit diffuse large B-cell lymphoma: does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book 2015; doi: 10.14694/EdBook_AM.2015.35.e449 e449–e457.
Camicia R, Winkler HC, Hassa PO . Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review. Mol Cancer 2015; 14: 207.
Acknowledgements
We thank the LYSARC for coordinating the study; Fabienne Morand, Sami Boussetta, Marion Fournier, Laurence Girard, Clemence Capron and the project leaders from the different countries; the patients and their families; AJE for reviewing the English language manuscript; Catherine Druon for preparing the manuscript; and all investigators and pathologists. Preliminary results presented at the 57th ASH 2015 annual meeting (abstract #731). Written on behalf of CORAL (Collaborative trial in Relapsed Aggressive Lymphoma).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EVDN: Roche: Travel accommodation. CG: Roche: Research funding. MT: Roche: Honoraria, research funding. JR: Roche: Consultancy. UD: Roche Pharma: Honoraria, research funding. CHM: Genentech: Consultancy, research funding. BG: Roche: Honoraria, research funding.
Rights and permissions
About this article
Cite this article
Van Den Neste, E., Schmitz, N., Mounier, N. et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant 52, 216–221 (2017). https://doi.org/10.1038/bmt.2016.213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.213
This article is cited by
-
Identifying effect modifiers of CAR-T cell therapeutic efficacy: a systematic review and individual patient data meta-analysis protocol
Systematic Reviews (2023)
-
Recent advances in CAR T-cell therapy for lymphoma in China
Clinical and Translational Oncology (2023)
-
Salvage Using Polatuzumab Vedotin Based Therapy in Relapsed Refractory Large B-Cell Lymphomas: Early Experience from a Real-World Middle-Income Setting Using Named-Patient Compassionate Access Program
Indian Journal of Hematology and Blood Transfusion (2023)
-
Identification of the estrogen receptor beta as a possible new tamoxifen-sensitive target in diffuse large B-cell lymphoma
Blood Cancer Journal (2022)
-
Polatuzumab vedotin–based salvage immunochemotherapy as third-line or beyond treatment for patients with diffuse large B-cell lymphoma: a real-world experience
Annals of Hematology (2022)