Abstract
A record number of 40 829 hematopoietic stem cell transplantation (HSCT) in 36 469 patients (15 765 allogeneic (43%), 20 704 autologous (57%)) were reported by 656 centers in 47 countries to the 2014 survey. Trends include: continued growth in transplant activity, more so in Eastern European countries than in the west; a continued increase in the use of haploidentical family donors (by 25%) and slower growth for unrelated donor HSCT. The use of cord blood as a stem cell source has decreased again in 2014. Main indications for HSCT were leukemias: 11 853 (33%; 96% allogeneic); lymphoid neoplasias; 20 802 (57%; 11% allogeneic); solid tumors; 1458 (4%; 3% allogeneic) and non-malignant disorders; 2203 (6%; 88% allogeneic). Changes in transplant activity include more allogeneic HSCT for AML in CR1, myeloproliferative neoplasm (MPN) and aplastic anemia and decreasing use in CLL; and more autologous HSCT for plasma cell disorders and in particular for amyloidosis. In addition, data on numbers of teams doing alternative donor transplants, allogeneic after autologous HSCT, autologous cord blood transplants are presented.
Similar content being viewed by others
Introduction
Hematopoietic stem cell transplantation (HSCT) is an established procedure for many acquired and congenital disorders of the hematopoietic system, including disorders of the immune system, and as enzyme replacement in metabolic disorders.1, 2, 3, 4 The annual activity survey of the European Society of Blood and Marrow Transplantation (EBMT), describing the status of HSCT in Europe and affiliated countries, has become an instrument used to observe trends and to monitor changes in technology use.5, 6, 7, 8, 9, 10, 11, 12 The survey captures the numbers of HSCT performed in the preceding year from each participating team, divided by indication, donor type and stem cell source. The standardized structure of the survey over many years and the excellent commitment of the participating teams allow us to observe changes over time and to evaluate factors associated with these changes. More recently, the survey has included additional information on novel cell therapies with hematopoietic stem cells for non-hematopoietic use, as well as on the use of non-hematopoietic stem and progenitor cells. This coincides with the recent interest of the World Health Organization (WHO) (www.who.org) in cell and tissue transplants, and further stresses the need for adequate and timely information.13 The analysis of the survey data spanning over 20 years has shown a continued and constant increase in the annual numbers of HSCT and transplant rates (number of HSCT per 10 million inhabitants) for both allogeneic and autologous HSCT.
This report is based on the 2014 survey data. In addition to transplant rates and indications, this report focuses on the use of donors other than HLA identical siblings and matched unrelated donors for allogeneic HSCT.
Patients and methods
Data collection and validation
Participating teams were invited to report data for 2014 by indication, stem cell source and donor type as listed in Table 1. The survey allows the possibility to report additional information on the numbers of subsequent transplants performed as a result of relapse, rejection or those that are part of a planned sequential transplant protocol. Supplementary information on the numbers of donor lymphocyte infusions, reduced intensity HSCT and the numbers of pediatric HSCT is also collected. Quality control measures included several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT Registry database and cross-checking with the National Registries.
Teams
In all, 680 centers from 49 countries were contacted for the 2014 survey (40 European and 9 affiliated countries); of which 656 teams reported. This corresponds to a 97% return rate and includes 553 active EBMT member teams. Twenty-six active teams failed to report in 2014.
Contacted teams are listed in the Supplementary information in alphabetical order by country, city, EBMT centre code, with their reported numbers of first and total HSCT, and of first allogeneic and autologous HSCT. The WHO regional office definitions (www.who.org) were used to classify countries as European or Non-European. Nine non-European countries participated in the 2014 EBMT survey: Algeria, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa and Tunisia. Their data (2716 HSCT) from 30 actively transplanting teams makes up 6.7% of the total data set and are included in all analyses.
Patient and transplant numbers
Wherever appropriate, patient numbers corresponding to the number of patients receiving a first transplant and transplant numbers reflecting the total number of transplants performed are listed.
The term sibling donor includes HLA identical siblings and twins but not siblings with HLA mismatches. Unrelated donor transplants include HSCT from unrelated donors with peripheral blood and marrow as a stem cell source but not cord blood HSCT, these are shown as cord blood HSCT in Figure 3a. Mismatched family donors are termed ‘haploidentical’ for the purpose of this analysis, but this category includes also mismatched related donors that are mismatched to a lesser degree than a full haplotype. As the haplotype mismatched donors are the majority in this category, the term ‘haploidentical’ is used for the entire group.
Multiple transplants may include multiple transplants defined as subsequent transplants within a planned double or triple autologous or allogeneic transplant protocol, and retransplants (autologous or allogeneic) defined as unplanned HSCT for rejection or relapse after a previous HSCT.
Transplant rates
Transplant rates, defined as the total number of HSCT per 10 million inhabitants, were computed for each country without adjustments for patients who crossed borders and received their HSCT in a foreign country. Population numbers were obtained from Eurostats for 2014 for the European countries (http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database), and the US census bureau database for the non-European countries (http://www.census.gov/population/international/data/idb/rank.php).
Analysis
Wherever appropriate absolute numbers of transplanted patients, transplants or transplant rates are shown for specific countries, indications or transplant techniques.
Results
2014 Data
Participating teams in 2014
Of the 656 teams, 410 (62%) performed both allogeneic and autologous transplants; 227 (35%) restricted their activity to autologous HSCT, and 13 teams (2%) to allogeneic transplants only. Six teams (1%) reported having performed no transplants in 2014 due to renovation or temporary closure of the transplant unit. Of the 656 active centers, 118 (18%) centers performed transplants on both adult and pediatric patients. An additional 107 (16%) centers were dedicated pediatric transplant centers and 431 (66%) centers performed transplants on adults only.
Numbers of patients and transplants
In 2014, 40 829 transplants were reported in 36 469 patients (first transplant) increasing the number of transplants to over 40 000 for the first time since 1990. Of these, 16 946 (42%) were allogeneic; 23 883 (58%) were autologous. When compared with 2013, the total number of transplants increased by 4.1% (4.5% allogeneic HSCT and 3.8% autologous HSCT).11 Furthermore, there were 4360 second or subsequent transplants 1181 allogeneic and 3179 autologous. The total number of transplants increased by 22% compared with 5 years (since 2010) and 80% compared with 15 years (2000) previously. The total number of patients transplanted under the age of 18 in both dedicated and joint adult-pediatric units was 4400 (3279 allogeneic and 1121 autologous HSCT). Of these, 3117 patients (71%) (2420 allogenic and 697 autologous) were performed in dedicated pediatric centers.
Indications
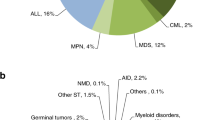
Indications for HSCT in 2014 are listed in detail in Table 1. Main indications were leukemia’s (including primarily AML, ALL and myelodysplastic syndrome (MDS)/myelodysplastic (MD)/myeloproliferative neoplasm (MPN)): 11 853 (33% of total; 96% of which were allogeneic); lymphoid neoplasias including non-Hodgkin lymphoma (NHL), Hodgkin's lymphoma and plasma cell disorders: 20 802 (57%; 11% allogeneic); solid tumors: 1458 (4%; 3% allogeneic); and non-malignant disorders; 2203 (6%; 88% allogeneic). As seen in previous years, the majority of HSCT for lymphoid malignancies were autologous while most transplants for leukemia were performed using stem cells from allogeneic donors. Autologous HSCT for non-malignant disorders predominantly include patients with autoimmune disorders (Figures 1a and b). For the first time since 1990 >50% of patients receiving autologous HSCT had plasma cell disorders (Figure 1b).
Distributions of indications for HSCT are shown in Figures 1a and b for allogeneic and autologous HSCT, respectively. Compared with 2013, there were increases in allogeneic HSCT for AML in CR1 by 13%, myeloproliferative neoplasm 14% and severe aplastic anemia (SAA) by 12%. A decrease was seen in CLL by 21%. For autologous HSCT, there was an increase in myeloma by 5%, amyloidosis by 44% (N=114), Hodgkin's lymphoma by 8% and autoimmune disease by 40%. No major decrease in activity was seen for any given disease. In all, 2649 patients received treatment with donor lymphocyte infusions, a 5.4% increase since 2013. In all, 6871 of the total allogeneic HSCT were performed using non-myeloablative conditioning. This is an increase of 5.2% since in 2014 and is 41% of all allogeneic HSCT.
Transplant rates
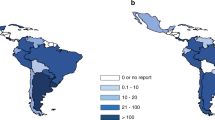
Figures 2a and b show transplant rates by country for allogeneic and autologous HSCT comparing rates in 2014. Median transplant rates per 10 million inhabitants were 133 (range, 5.2–495) for allogeneic HSCT and 230 (range, 9.5–530) for autologous HSCT in 2014. As shown in Figure 3a, numbers of allogeneic HSCT continue to increase, more in unrelated donor HSCT than in sibling HSCT, while cord blood transplants are slightly decreasing. There is a significant increase in transplants from haploidentical donors and it appears that this comes at the expense of a slower increase in the use of unrelated donors and cord blood.14, 15, 16
As these transplants are done by dedicated teams, Figure 3b shows the development of the number of teams since 1990 reporting any type of HSCT. The figure shows that the increase in haploidentical HSCT is paralleled by the number of teams performing these types of HSCT and similarly a lower number of teams reporting cord blood HSCT in 2014.
The activity survey has collected data on allogeneic HSCT in patients having had a prior autologous HSCT either planned or to treat relapse. Figure 4 shows 566 allogeneic transplants after an autograft treatment protocol. The main indications were myeloma (39% N=223), NHL (30% N=171) and Hodgkin's lymphoma (20% N=114). The numbers have remained more or less stable over the last 10 years.
Autologous cord blood transplantation
Information on cord blood transplants has been collected since this stem cell source was introduced in 1997. As numerous autologous cord blood banks exist, the use of autologous cord blood is of interest.17 We found since 2007 a small number of confirmed autologous cord blood HSCT. The main indication was SAA (N=6) followed by NHL (N=3), neuroblastoma (N=3), MDS (N=2), MPN (N=1) and other solid tumor (N=1).
Evolving countries
HSCT activity is increasing in many countries; in some countries, this increase was particularly striking; in particular a clear growth in transplant activity in countries of Eastern Europe can be seen. Figures 5a and b show increases in % of allogeneic and autologous transplant activity respectively since 2004, that is, for the last 10 years in all countries reporting >100 HSCT in 2014. The greatest increase was seen for allogeneic HSCT in Romania, Russia, Turkey, Croatia, Lithuania and Serbia (ranging from 1000% to >200%, respectively) and for autologous HSCT in Romania, Serbia, Russia, Turkey and Iran (ranging from 1200% to >200%, respectively).
Additional cellular therapies
In all, 15 teams from 11 countries reported through the activity survey the treatment of 160 patients with hematopoietic stem cells for non-hematopoietic use in 2014. A total of 152 therapies were performed using autologous HSCs with 8 using allogeneic HSCs for epithelial diseases. The main indications were cardiovascular, 88; neurological, 47; tissue repair, 17 and epithelial: 8. In addition, 525 patients in 93 teams and 20 countries received mesenchymal stromal cells for prevention/treatment of GvHD (450, increasing from 213 in 2010 and 344 in 2013), prevention/treatment of graft failure (27) and for unspecified reasons (48; 38 of which were allogeneic).
Discussion
The EBMT activity survey has been conducted annually since 1990.6 The 2010 survey reported for the first time more than 30 000 patients transplanted in a given year,18 this is now followed by >40 000 transplants in this 2014 survey. Therefore, transplant numbers continue to increase unabated.
HSCT for some indications continues to increase but not for others. Of interest is growth of allogeneic HSCT for AML in CR1, myeloproliferative neoplasia and marrow failure and a notable decrease in CLL possibly due to the availability of novel kinase inhibitors. In autologous HSCT, transplant activity in myeloma continues to increase and in particular in amyloidosis possibly following publication of positive results.19
In allogeneic HSCT, the use of unrelated donors continues to increase but less so than in previous years (median 12% annual increase in the years 2004–2010 and 5% annual increase in the years 2011–2014). This is compensated for by an increase in the use of haploidentical donors, who are in some sense competition for unrelated and cord blood transplants (median 2% annual increase in the years 2004–2010 and 25% annual increase in the years 2011–2014).
In this year's survey, we looked at the number of teams performing these particular transplants and activity in unrelated donor cord blood HSCT and haploidentical HSCTs mirrors the transplant activity for these particular indications, that is, more haploidentical HSCTs are also performed by more teams doing haploidentical HSCT.
We looked for the first time at allogeneic HSCTs done after autologous HSCTs. The majority of these transplants are for myeloma, non-Hodgkin lymphoma and Hodgkin's lymphoma showing that probably the majority of this activity reflects salvage allogeneic procedures after failed autologous HSCT.
Transplant activity is distributed unevenly throughout Europe, and several analyses have shown correlation with income. As shown here the majority in increased activity for allogeneic and autologous HSCT are in middle income countries, particularly in Eastern Europe who are catching up with Western European countries.
We have added a paragraph on the use of autologous cord blood transplantation. These cord blood products were most certainly retrieved from private autologous cord blood banks.17 The largest indication was for marrow failure. The numbers shown here, that is, slightly more than two procedures per year reflect the rare indications for such transplants and may fuel the discussion on the usefulness of autologous cord blood banks in Europe.
In conclusion, this year's activity survey shows a continued increase in the use of HSCT across Europe. Some trends are visible and are discussed here. The paper reflects current practice and results may be useful to health-care planning and health policy makers.
References
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Appelbaum FR . Hematopoietic-cell transplantation at 50. N Engl J Med 2007; 357: 1472–1475.
Ljungman P, Bregni M, Brune M, Cornelissen J, deWitte T, Dini G et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219–234.
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624.
Alois G, Marcelo CP, Mahmoud A, Yoshiko A, Helen B, Lydia F et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol 2015; 2: e91–e100.
Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Frauendorfer K et al. The EBMT activity survey 2008 impact of team size, team density and new trends. Bone Marrow Transplant 2011; 46: 174–191.
Gratwohl A . Bone marrow transplantation activity in Europe 1990. Report from the European Group for Bone Marrow Transplantation (EBMT). Bone Marrow Transplant 1991; 8: 197–201.
Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A . Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT). Current trends in haematopoietic stem cell transplantation in Europe. Blood 2002; 100: 2374–2386.
Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant 2009; 43: 275–291.
Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J, Niederwieser D et al. Changes in use of hematopoietic stem cell transplantation; a model for diffusion of medical technology. Haematologica 2010; 95: 637–643.
Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant 2014; 49: 744–750.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant 2015; 50: 476–482.
World Health Organisation, WHO (http://www.who.int/topics/transplantation/en/).
Foeken LM, Green A, Hurley CK, Marry E, Wiegand T, Oudshoorn M . Monitoring the international use of unrelated donors for transplantation: the WMDA annual reports. Bone Marrow Transplant 2010; 45: 811–818.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118: 282–288.
Luznik L, O'Donnell PV, Symons, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Ballen KK, Verter F, Kurtzberg J . Umbilical cord blood donation: public or private? Bone Marrow Transplant 2015; 50: 1271–1278.
Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant 2012; 47: 906–923.
Anita D’Souza, Angela D, Baldeep W, Mei-Jie Z, Jiaxing H, Morie AG et al. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a center for International blood and marrow transplant research study. J Clin Oncol 2015; 33: 3741–3749.
Acknowledgements
The cooperation of all participating teams and their staff (listed in the Supplementary information), the EBMT Co-ordination offices; Barcelona, Paris, London (C Ruiz de Elvira), the Austrian Registry (ASCTR) (H Greinix, B Lindner, C Wagner), the Belgium Registry (Yves Beguin, M Van Spauwen) the Czech Registry (P Zak, M Trnkova, K Benesova), the French Registry (SFGM) (I Yakoub-Agha, N Raus), the German Registry (DRST) (H Ottinger, K Fuchs, C Müller, H Neidlinger, F Hanke), the Italian Registry (GITMO) (F Bonifazi, B Bruno, E Oldani), the Dutch Registry (JJ Cornelissen, M Groenendijk), the Spanish Registry (GETH) (J Diez Martin, A Cedillo), the Swiss Registry (SBST) (U Schanz, H Baldomero), the Turkish Registry (G Gurman, M Arat) and the British Registry (BSBMT) (K Kirkland, J Perry) is greatly appreciated. We also thank D John for database support. EBMT is supported by grants from the corporate sponsors: Jazz Pharmaceuticals plc, Molmed S.p.A, AstellasPharma Europe Ltd, Celgene International SARL, Clinigen Group Ltd, Gilead Sciences Europe Ltd., GlaxoSmithKline plc., Hospira Inc., Janssen, Medac Hematology GmbH, MiltenyiBiotec GmbH, MSD Sharp&Dohme GmbH, Neovii Biotech GmbH, Pfizer Oncology, Sanofi Oncology, Takeda Pharmaceuticals, Therakos Photopheresis, Alexion, Amgen Oncology GmbH, Kiadis Pharma, Macropharma, Mundipharma, Pierre Fabre Médicament and Terumo BCT.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Passweg, J., Baldomero, H., Bader, P. et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant 51, 786–792 (2016). https://doi.org/10.1038/bmt.2016.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.20
This article is cited by
-
Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT
Bone Marrow Transplantation (2024)
-
Long-term follow-up of patients with acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation after primary induction failure
Blood Cancer Journal (2023)
-
Multi-parametric MRI in the diagnosis and scoring of gastrointestinal acute graft-versus-host disease
European Radiology (2023)
-
Hydrogel-based microenvironment engineering of haematopoietic stem cells
Cellular and Molecular Life Sciences (2023)
-
Survival protection of patients undergoing hematopoietic stem cell transplantation: grounded theory
Supportive Care in Cancer (2023)