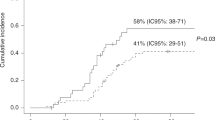
Abstract
Pneumocystis jiroveci pneumonia (PJP) is associated with high morbidity and mortality after hematopoietic stem cell transplantation (HSCT). Little is known about PJP infections after HSCT because of the rarity of disease given routine prophylaxis. We report the results of a Center for International Blood and Marrow Transplant Research study evaluating the incidence, timing, prophylaxis agents, risk factors and mortality of PJP after autologous (auto) and allogeneic (allo) HSCT. Between 1995 and 2005, 0.63% allo recipients and 0.28% auto recipients of first HSCT developed PJP. Cases occurred as early as 30 days to beyond a year after allo HSCT. A nested case cohort analysis with supplemental data (n=68 allo cases, n=111 allo controls) revealed that risk factors for PJP infection included lymphopenia and mismatch after HSCT. After allo or auto HSCT, overall survival was significantly poorer among cases vs controls (P=0.0004). After controlling for significant variables, the proportional hazards model revealed that PJP cases were 6.87 times more likely to die vs matched controls (P<0.0001). We conclude PJP infection is rare after HSCT but is associated with high mortality. Factors associated with GVHD and with poor immune reconstitution are among the risk factors for PJP and suggest that protracted prophylaxis for PJP in high-risk HSCT recipients may improve outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gilroy SA, Bennett NJ . Pneumocystis pneumonia. Semin Respir Crit Care Med 2011; 32: 775–782.
Wakefield AE, Peters SE, Banerji S, Bridge PD, Hall GS, Hawksworth DL et al. Pneumocystis carinii shows DNA homology with the ustomycetous red yeast fungi. Mol Microbiol 1992; 6: 1903–1911.
Gluck T, Geerdes-Fenge HF, Straub RH, Raffenberg M, Lang B, Lode H et al. Pneumocystis carinii pneumonia as a complication of immunosuppressive therapy. Infection 2000; 28: 227–230.
Tuan IZ, Dennison D, Weisdorf DJ . Pneumocystis carinii pneumonitis following bone marrow transplantation. Bone Marrow Transplant 1992; 10: 267–272.
Wazir JF, Ansari NA . Pneumocystis carinii infection. Update and review. Arch Pathol Lab Med 2004; 128: 1023–1027.
Torres HA, Chemaly RF, Storey R, Aguilera EA, Noqueras GM, Safdar A et al. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jiroveci pneumonia in cancer patients. Eur J Clin Microbiol Infect Dis 2006; 25: 382–388.
Yoo JH, Lee DG, Choi SM, Choi JH, Park YH, Kim YJ et al. Infectious complications and outcomes after allogeneic hematopoietic stem cell transplantation in Korea. Bone Marrow Transplant 2004; 34: 497–504.
Rodriguez M, Fishman JA . Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 2004; 17: 770–782.
Tasaka S, Tokuda H . Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother 2012; 18: 793–806.
Green H, Paul M, Vidal L, Leibovici L . Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2007; 3: CD005590.
De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, Gluckman E et al. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant 2005; 36: 879–883.
Mikaelsson L, Jacobsson G, Andersson R . Pneumocystis pneumonia—a retrospective study 1991-2001 in Gothenburg, Sweden. J Infect 2006; 53: 260–265.
Kruger W, Russmann B, Kroger N, Salomon C, Ekopf N, Elsner HA et al. Early infections in patients undergoing bone marrow or blood stem cell transplantation—a 7 year single centre investigation of 409 cases. Bone Marrow Transplant 1999; 23: 589–597.
Tomonari A, Takahashi S, Ooi J, Tsukada N, Konuma T, Kato S et al. No occurrence of Pneumocystis jiroveci (carinii) pneumonia in 120 adults undergoing myeloablative unrelated cord blood transplantation. Transpl Infect Dis 2008; 10: 303–307.
Chapman JR, Marriott DJ, Chen SC, MacDonald PS . Post-transplant Pneumocystis jirovecii pneumonia—a re-emerged public health problem? Kidney Int 2013; 84: 240–243.
Nankivell BJ, Firacative C, Kable K, Chen SC, Meyer W . Molecular epidemiology linking multihospital clusters of opportunistic Pneumocystis jirovecii pneumonia. Clin Infect Dis 2013; 57: 1058–1059.
Morris A, Norris KA . Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 2012; 25: 297–317.
Dykewicz CA . Preventing opportunistic infections in bone marrow transplant recipients. Transpl Infect Dis 1999; 1: 40–49.
Fontanet ACY, Roosnek E, Mohty B, Passweg JR . Cotrimoxazole myelotoxicity in hematopoietic SCT recipients: time for reappraisal. Bone Marrow Transplant 2011; 46: 1272–1273.
Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T . A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1999; 24: 897–902.
Fishman JA . Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis 2001; 33: 1397–1405.
Sangiolo D, Storer B, Nash R, Corey L, Davis C, Flowers M et al. Toxicity and efficacy of daily dapsone as Pneumocystis jiroveci prophylaxis after hematopoietic stem cell transplantation: a case-control study. Biol Blood Marrow Transplant 2005; 11: 521–529.
Marras TK, Sanders K, Lipton JH, Messner HA, Conly J, Chan CK . Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transpl Infect Dis 2002; 4: 66–74.
Ramesh M, Chandrasekar PH . Effective alternates to trimethoprim-sulfamethoxazole as antimicrobial prophylaxis in stem cell recipients: are there any? Pediatr Transplant 2008; 12: 823–826.
Utili R, Durante-Mangoni E, Basilico C, Mattei A, Ragone E, Grossi P . Efficacy of caspofungin addition to trimethoprim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation 2007; 84: 685–688.
Rodriguez M, Sifri CD, Fishman JA . Failure of low-dose atovaquone prophylaxis against Pneumocystis jiroveci infection in transplant recipients. Clin Infect Dis 2004; 38: e76–e78.
Maltezou HC, Petropoulos D, Choroszy M, Gardner M, Mantzouranis EC, Rolston KV et al. Dapsone for Pneumocystis carinii prophylaxis in children undergoing bone marrow transplantation. Bone Marrow Transplant 1997; 20: 879–881.
Vasconcelles MJ, Bernardo MV, King C, Weller EA, Antin JH . Aerosolized pentamidine as pneumocystis prophylaxis after bone marrow transplantation is inferior to other regimens and is associated with decreased survival and an increased risk of other infections. Biol Blood Marrow Transplant 2000; 6: 35–43.
Souza JP, Boeckh M, Gooley TA, Flowers ME, Crawford SW . High rates of Pneumocystis carinii pneumonia in allogeneic blood and marrow transplant recipients receiving dapsone prophylaxis. Clin Infect Dis 1999; 29: 1467–1471.
Afessa B, Peters SG . Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med 2006; 27: 297–309.
Gea-Banacloche J, Masur H, Arns da Cunha C, Chiller T, Kirchhoff LV, Shaw P et al. Regionally limited or rare infections: prevention after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44: 489–494.
Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D . Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 1992; 267: 832–837.
Russian DA, Levine SJ . Pneumocystis carinii pneumonia in patients without HIV infection. Am J Med Sci 2001; 321: 56–65.
Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 2014; 44: 185–198.
Worth LJ, Dooley MJ, Seymour JF, Mileshkin L, Slavin MA, Thursky KA . An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. Br J Cancer 2005; 92: 867–872.
Ahuja J, Kanne JP . Thoracic infections in immunocompromised patients. Radiol Clin North Am 2014; 52: 121–136.
Saito T, Seo S, Kanda Y, Shoji N, Ogasawara T, Murakami J et al. Early onset Pneumocystis carinii pneumonia after allogeneic peripheral blood stem cell transplantation. Am J Hematol 2001; 67: 206–209.
Bjorklund A, Aschan J, Labopin M, Remberger M, Ringden O, Winiarski J et al. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 40: 1055–1062.
Roblot F, Le Moal G, Kauffmann-Lacroix C, Bastides F, Boutoille D, Verdon R et al. Pneumocystis jirovecii pneumonia in HIV-negative patients: a prospective study with focus on immunosuppressive drugs and markers of immune impairment. Scand J Infect Dis 2014; 46: 210–214.
Lee EW, Wei LJ, Amato DA Cox-type regression analysis for large numbers of small groups of correlated failure time observations". In: Klein JP and Goel PK (eds). Survival Analysis: State of the Art. Kluwer Academic Publishers: Dordrecht, Netherlands, 1992 pp 237–247.
Olsson M, Eriksson BM, Elvin K, Strandberg M, Wahlgren M . Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scand J Infect Dis 2001; 33: 285–289.
Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A . Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-beta-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol 2013; 51: 3380–3388.
Esteves F, Lee CH, de Sousa B, Badura R, Seringa M, Fernandes C et al. (1-3)-Beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis 2014; 33: 1173–1180.
Fan LC, Lu HW, Cheng KB, Li HP, Xu JF . Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PloS ONE 2013; 8: e73099.
Maillet M, Maubon D, Brion JP, Francois P, Molina L, Stahl JP et al. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. Eur J Clin Microbiol Infect Dis 2014; 33: 331–336.
Matsumura Y, Ito Y, Yamamoto M, Matsushima A, Nagao M, Takakura S et al. Pneumocystis polymerase chain reaction and blood (1—>3)-beta-D-glucan assays to predict survival with suspected Pneumocystis jirovecii pneumonia. J Infect Chemother 2014; 20: 109–114.
Olsson M, Stralin K, Holmberg H . Clinical significance of nested polymerase chain reaction and immunofluorescence for detection of Pneumocystis carinii pneumonia. Clin Microbiol Infect 2001; 7: 492–497.
Tasaka S, Tokuda H . Recent advances in the diagnosis of Pneumocystis jirovecii pneumonia in HIV-infected adults. Expert Opin Med Diagn 2013; 7: 85–97.
Coyle PV, McCaughey C, Nager A, McKenna J, O'Neill H, Feeney SA et al. Rising incidence of Pneumocystis jirovecii pneumonia suggests iatrogenic exposure of immune-compromised patients may be becoming a significant problem. J Med Microbiol 2012; 61: 1009–1015.
Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V et al. Increasing Pneumocystis pneumonia, England, UK, 2000-2010. Emerg Infect Dis 2013; 19: 386–392.
Madden RM, Pui CH, Hughes WT, Flynn PM, Leung W . Prophylaxis of Pneumocystis carinii pneumonia with atovaquone in children with leukemia. Cancer 2007; 109: 1654–1658.
Meyers B, Borrego F, Papanicolaou G . Pneumocystis carinii pneumonia prophylaxis with atovaquone in trimethoprim-sulfamethoxazole-intolerant orthotopic liver transplant patients: a preliminary study. Liver Transpl 2001; 7: 750–751.
Shankar SM, Nania JJ . Management of Pneumocystis jiroveci pneumonia in children receiving chemotherapy. Paediatr Drugs 2007; 9: 301–309.
Neumann S, Krause SW, Maschmeyer G, Schiel X, von Lilienfeld-Toal M . Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematological malignancies and solid tumors: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2013; 92: 433–442.
Overgaard UM, Helweg-Larsen J . Pneumocystis jiroveci pneumonia (PCP) in HIV-1-negative patients: a retrospective study 2002-2004. Scand J Infect Dis 2007; 39: 589–595.
Williams KM, Hakim FT, Gress RE . T cell immune reconstitution following lymphodepletion. Semin Immunol 2007; 19: 318–330.
Kim SY, Dabb AA, Glenn DJ, Snyder KM, Chuk MK, Loeb DM . Intravenous pentamidine is effective as second line Pneumocystis pneumonia prophylaxis in pediatric oncology patients. Pediatr Blood Cancer 2008; 50: 779–783.
Debs RJ, Straubinger RM, Brunette EN, Lin JM, Lin EJ, Montgomery AB et al. Selective enhancement of pentamidine uptake in the lung by aerosolization and delivery in liposomes. Am Rev Respir Dis 1987; 135: 731–737.
Williams S, MacDonald P, Hoyer JD, Barr RD, Athale UH . Methemoglobinemia in children with acute lymphoblastic leukemia (ALL) receiving dapsone for pneumocystis carinii pneumonia (PCP) prophylaxis: a correlation with cytochrome b5 reductase (Cb5R) enzyme levels. Pediatr Blood Cancer 2005; 44: 55–62.
Queener SF, Cody V, Pace J, Torkelson P, Gangjee A . Trimethoprim resistance of dihydrofolate reductase variants from clinical isolates of Pneumocystis jirovecii. Antimicrob Agents Chemother 2013; 57: 4990–4998.
Caselli D, Petris MG, Rondelli R, Carraro F, Colombini A, Muggeo P et al. Single-day trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis pneumonia in children with cancer. J Pediatr 2014; 164: 389–392 e1.
Link H, Vohringer HF, Wingen F, Bragas B, Schwardt A, Ehninger G . Pentamidine aerosol for prophylaxis of Pneumocystis carinii pneumonia after BMT. Bone Marrow Transplant 1993; 11: 403–406.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Williams, K., Ahn, K., Chen, M. et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant 51, 573–580 (2016). https://doi.org/10.1038/bmt.2015.316
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.316
This article is cited by
-
Negative serum (1,3) -β-D-glucan has a low power to exclude Pneumocystis jirovecii pneumonia (PJP) in HIV-uninfected patients with positive qPCR
Annals of Clinical Microbiology and Antimicrobials (2023)
-
Incidence and risk factors of opportunistic infections after autologous stem cell transplantation: a nationwide, population-based cohort study in Korea
Scientific Reports (2023)
-
Pneumocystis jirovecii Infection in autologous hematopoietic stem cell transplant recipients
Bone Marrow Transplantation (2023)
-
Effectiveness and tolerability of intravenous pentamidine for Pneumocystis carinii pneumonia prophylaxis in adult hematopoietic stem cell transplant patients: a retrospective study
BMC Infectious Diseases (2020)
-
Low B cell counts as risk factor for infectious complications in systemic sclerosis after autologous hematopoietic stem cell transplantation
Arthritis Research & Therapy (2020)