Abstract
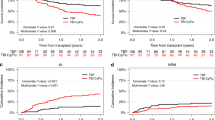
Unmanipulated haploidentical transplantation (Haplo-SCT) using post-transplantation cyclophosphamide (PT-Cy) represents an alternative for patients with high-risk diseases lacking HLA-identical donor. Although it provides low incidences of GVHD, the efficacy of Haplo-SCT is still questioned, especially for patients with myeloid malignancies. Thus, we analyzed 60 consecutive patients with refractory (n=30) or high-risk CR (n=30) AML or myelodysplastic syndromes (MDSs) who underwent PT-Cy Haplo-SCT. The median age was 57 years (22–73 years), hematopoietic cell transplantation comorbidity index was ⩾3 in 38 patients (63%) and Haplo-SCT was the second allogeneic transplantation for 10 patients (17%). Although most of patients received PBSC as graft source (n=48, 80%), we found low incidences of grade 3–4 acute (2%) and severe chronic GVHD (4%). Among patients with high-risk CR diseases, 1-year non-relapse mortality, cumulative incidence of relapse, progression-free and overall survivals were 20%, 32%, 47% and 62%, respectively. In patients with refractory disease, corresponding results were 34%, 35%, 32% and 37%, respectively. We conclude that PT-Cy Haplo-SCT could provide promising anti-leukemic effect even in the setting of very advanced diseases. Thus, it represents a viable alternative for high-risk AML/MDS patients without HLA-identical donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31: 1310–1316.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118: 282–288.
Raiola A, Dominietto A, Varaldo R, Ghiso A, Galaverna F, Bramanti S et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphoma. Bone Marrow Transplant 2014; 49: 190–194.
Garciaz S, Castagna L, Bouabdallah R, Fürst S, Bramanti S, Coso D et al. Familial haploidentical challenging unrelated donor Allo-SCT in advanced non-Hodgkin lymphomas when matched related donor is not available. Bone Marrow Transplant 2015; 50: 865–867.
Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008; 14: 1279–1287.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120: 2454–2465.
Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE . The analysis of failure times in the presence of competing risks. Biometrics 1978; 34: 541–554.
Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 1988; 16: 1141–1154.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Kaplan E, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Devillier R, Crocchiolo R, Castagna L, Fürst S, Cheikh JEl, Faucher C et al. The increase from 2.5 to 5 mg/kg of rabbit anti-thymocyte-globulin dose in reduced intensity conditioning reduces acute and chronic GVHD for patients with myeloid malignancies undergoing allo-SCT. Bone Marrow Transplant 2012; 47: 639–645.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite endpoint of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125: 133–138.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Castagna L, Crocchiolo R, Furst S, Bramanti S, Cheikh JEl, Sarina B et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant 2014; 20: 724–729.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb H-J . Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 2005; 23: 5675–5687.
Krejci M, Doubek M, Dusek J, Brychtova Y, Racil Z, Navratil M et al. Combination of fludarabine, amsacrine, and cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia. Ann Hematol 2013; 92: 1397–1403.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 2014; 32: 3497–3505.
Kanakry CG, Tsai H-L, Bolaños-Meade J, Smith BD, Gojo I, Kanakry JA et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014; 124: 3817–3827.
Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood 2015; 125: 2855–2864.
Noviello M, Forcina A, Veronica V, Crocchiolo R, Stanghellini MTL, Carrabba M et al. Early recovery of CMV immunity after HLA-haploidentical hematopoietic stem cell transplantation as a surrogate biomarker for a reduced risk of severe infections overall. Bone Marrow Transplant 2015; 50: 1262–1264.
Oudin C, Chevallier P, Furst S, Guillaume T, Cheikh JEl, Delaunay J et al. Reduced-toxicity conditioning prior to allogeneic stem cell transplantation improves outcome in patients with myeloid malignancies. Haematologica 2014; 99: 1762–1768.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013; 19: 117–122.
Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant 2014; 20: 1975–1981.
Tischer J, Stemmler HJ, Engel N, Hubmann M, Fritsch S, Prevalsek D et al. Feasibility of clofarabine cytoreduction followed by haploidentical hematopoietic stem cell transplantation in patients with relapsed or refractory advanced acute leukemia. Ann Hematol 2013; 92: 1379–1388.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant 2015; 50: S37–S39.
Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015; 126: 1033–1040.
Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant 2010; 45: 429–436.
Wang Y, Liu D-H, Liu K-Y, Xu L-P, Zhang X-H, Han W et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119: 978–985.
Wang Y, Liu D-H, Liu K-Y, Xu L-P, Zhang X-H, Han W et al. Impact of pretransplantation risk factors on post transplantation outcome of patients with acute myeloid leukemia in remission after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19: 283–290.
Yahng S-A, Kim J-H, Jeon Y-W, Yoon J-H, Shin S-H, Lee S-E et al. A well-tolerated regimen of 800cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant 2015; 21: 119–129.
Shin S-H, Kim J-H, Jeon Y-W, Yoon J-H, Yahng S-A, Lee S-E et al. Feasible outcomes of T cell-replete haploidentical stem cell transplantation with reduced-intensity conditioning in patients with myelodysplastic syndrome. Biol Blood Marrow Transplant 2015; 21: 342–349.
Acknowledgements
We thank the nursing staff for providing excellent care for our patients and the ‘Association pour la Recherche contre le Cancer’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Devillier, R., Bramanti, S., Fürst, S. et al. T-replete haploidentical allogeneic transplantation using post-transplantation cyclophosphamide in advanced AML and myelodysplastic syndromes. Bone Marrow Transplant 51, 194–198 (2016). https://doi.org/10.1038/bmt.2015.270
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.270
This article is cited by
-
Peripheral blood haploidentical hematopoietic cell transplantation for patients aged 70 years and over with acute myeloid leukemia or high-risk myelodysplastic syndrome
Bone Marrow Transplantation (2024)
-
Unmanipulated haploidentical hematopoietic stem cell transplantation using very low-dose antithymocyte globulin and methylprednisolone in adults with relapsed/refractory acute leukemia
Annals of Hematology (2020)
-
Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients
Bone Marrow Transplantation (2019)
-
Myeloablative conditioning regimens with combined of haploidentical and cord blood transplantation for myelodysplastic syndrome patients
Bone Marrow Transplantation (2018)
-
Haplo-identical allografting with post-transplant cyclophosphamide in high-risk patients
Annals of Hematology (2018)