Abstract
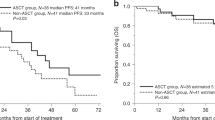
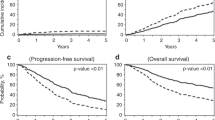
In patients with multiple myeloma (MM) undergoing autologous hematopoietic cell transplantation (auto-HCT), peripheral blood progenitor cells may be collected following mobilization with growth factor alone (GF) or cytotoxic chemotherapy plus GF (CC+GF). It is uncertain whether the method of mobilization affects post-transplant outcomes. We compared these mobilization strategies in a retrospective analysis of 968 patients with MM from the Center for International Blood and Marrow Transplant Research database who received an auto-HCT in the US and Canada between 2007 and 2012. The kinetics of neutrophil engraftment (⩾0.5 × 109/L) was similar between groups (13 vs 13 days, P=0.69) while platelet engraftment (⩾20 × 109/L) was slightly faster with CC+GF (19 vs 18 days, P=0.006). Adjusted 3-year PFS was 43% (95% confidence interval (CI) 38–48) in GF and 40% (95% CI 35–45) in CC+GF, P=0.33. Adjusted 3-year OS was 82% (95% CI 78–86) vs 80% (95% CI 75–84), P=0.43 and adjusted 5-year OS was 62% (95% CI 54–68) vs 60% (95% CI 52–67), P=0.76, for GF and CC+GF, respectively. We conclude that MM patients undergoing auto-HCT have similar outcomes irrespective of the method of mobilization and found no evidence that the addition of chemotherapy to mobilization contributes to disease control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 1998; 92: 3131–3136.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371: 895–905.
Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant 1997; 20: 211–217.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S . Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia 2008; 22: 1280–1281 author reply 1281-2.
Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia 2008; 22: 1282–1284.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 718–723.
Nazha A, Cook R, Vogl DT, Mangan PA, Gardler M, Hummel K et al. Stem cell collection in patients with multiple myeloma: impact of induction therapy and mobilization regimen. Bone Marrow Transplant 2011; 46: 59–63.
Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant 2008; 14: 795–798.
Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma 2006; 6: 384–388.
Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, Macpherson J, Winkler K et al. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biol Blood Marrow Transplant 2011; 17: 729–736.
Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C et al. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant 2011; 46: 523–528.
Schwartzberg LS, Birch R, Hazelton B, Tauer KW, Lee P Jr, Altemose R et al. Peripheral blood stem cell mobilization by chemotherapy with and without recombinant human granulocyte colony-stimulating factor. J Hematother 1992; 1: 317–327.
Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol 1998; 16: 1547–1553.
Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant 2008; 14: 1045–1056.
Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood 2001; 98: 2059–2064.
Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher 2014; 30: 176–182.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28: 1122–1128.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA December 6–9, 2014.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Uy, G., Costa, L., Hari, P. et al. Contribution of chemotherapy mobilization to disease control in multiple myeloma treated with autologous hematopoietic cell transplantation. Bone Marrow Transplant 50, 1513–1518 (2015). https://doi.org/10.1038/bmt.2015.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.190
This article is cited by
-
Chemotherapy-based versus chemotherapy-free stem cell mobilization (± plerixafor) in multiple myeloma patients: an Italian cost-effectiveness analysis
Bone Marrow Transplantation (2021)
-
Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G-CSF or G-CSF alone in multiple myeloma: a meta-analysis
Annals of Hematology (2021)
-
The association of mobilising regimen on immune reconstitution and survival in myeloma patients treated with bortezomib, cyclophosphamide and dexamethasone induction followed by a melphalan autograft
Bone Marrow Transplantation (2021)
-
Cost and efficacy of peripheral stem cell mobilization strategies in multiple myeloma
Bone Marrow Transplantation (2020)
-
Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement
Bone Marrow Transplantation (2019)